ABSTRACT
Objective: To determine the underdiagnosis rate in new COPD cases at the end of a nine-year follow-up period-in the study designated "Projeto Latino-Americano de Investigação em Obstrução Pulmonar" (PLATINO, Latin-American Pulmonary Obstruction Investigation Project)-and compare that with the underdiagnosis rate during the initial phase of the study, as well as to identify the clinical features exhibited by the subjects who were not diagnosed until the end of the follow-up phase. Methods: The study population comprised the 1,000 residents of the city of São Paulo, Brazil, who took part in the PLATINO study. Of those, 613 participated in the follow-up phase, during which the subjects were assessed with the same instruments and equipment employed in the initial phase of the study. We used the chi-square test or the independent sample t-test to analyze the underdiagnosis rate and to identify the characteristics of the subjects who were not diagnosed until the end of the follow-up phase. Results: The underdiagnosis rate for new COPD cases at the end of the nine-year follow-up period was 70.0%. The underdiagnosis rate during the follow-up phase was 17.5% lower than that reported for the initial phase of the study. The subjects who were not diagnosed until the end of the follow-up phase presented with fewer respiratory symptoms, better pulmonary function, and less severe disease than did those previously diagnosed with COPD. Conclusions: The underdiagnosis rate for new COPD cases was lower in the follow-up phase of the study than in the initial phase. The subjects who were not diagnosed until the end of the follow-up phase of the PLATINO study presented with the same clinical profile as did those who were not diagnosed in the initial phase. These findings underscore the need for spirometry in order to confirm the diagnosis of COPD and provide early intervention.
Keywords:
Pulmonary disease, chronic obstructive/diagnosis; Pulmonary disease, chronic obstructive/epidemiology; Spirometry.
RESUMO
Objetivo: Determinar a taxa de subdiagnóstico em novos casos de DPOC em uma amostra de pacientes após nove anos de seguimento do estudo "Projeto Latino-Americano de Investigação em Obstrução Pulmonar" (PLATINO) e compará-la à taxa de subdiagnóstico obtida na fase inicial do estudo, assim como identificar as características clínicas dos indivíduos subdiagnosticados na fase de seguimento. Métodos: A população desse estudo foi composta por 1.000 residentes na cidade de São Paulo que fizeram parte do estudo PLATINO. Desses, 613 indivíduos participaram da fase de seguimento. Os indivíduos foram avaliados utilizando-se os mesmos instrumentos e equipamentos na fase inicial do estudo. O teste do qui-quadrado ou o teste t para amostras independentes foi utilizado para analisar a taxa de subdiagnóstico e identificar as características dos indivíduos subdiagnosticados durante a fase de seguimento. Resultados: A taxa de subdiagnóstico para novos casos da DPOC após nove anos de acompanhamento foi de 70,0%. A taxa de subdiagnóstico na fase de seguimento foi 17,5% menor que a da fase inicial do estudo. Os indivíduos subdiagnosticados na fase de seguimento apresentavam poucos sintomas respiratórios, função pulmonar mais preservada e menor gravidade da doença do que aqueles previamente diagnosticados com DPOC. Conclusões: A taxa de subdiagnóstico na fase de seguimento foi menor que a da fase inicial do estudo. Os indivíduos subdiagnosticados na fase de seguimento do estudo PLATINO apresentavam o mesmo perfil clínico daqueles subdiagnosticados na fase inicial. Esses achados reforçam a necessidade da utilização da espirometria para o diagnóstico de DPOC e possibilitar a intervenção precoce.
Palavras-chave:
Doença pulmonar obstrutiva crônica/diagnóstico; Doença pulmonar obstrutiva crônica/epidemiologia; Espirometria.
IntroduçãoO Projeto Latino-Americano de Investigação em Obstrução Pulmonar (PLATINO) é um estudo epidemiológico de base populacional cujo objetivo principal foi investigar a prevalência de DPOC em cinco grandes cidades da América Latina.(1)
Na cidade de São Paulo, Brasil, a prevalência da DPOC foi de 15,8%.(2) A despeito de números tão grandiosos de prevalência, uma proporção pequena fora efetivamente diagnosticada. Apenas 12,5% dos portadores de DPOC diagnosticados por espirometria tinham um diagnóstico clínico já estabelecido da doença, ainda que um número significativo dos pacientes já apresentasse a doença sintomática bem definida.(3) O principal fator relacionado ao subdiagnóstico é a baixa utilização da espirometria como ferramenta diagnóstica.(4)
O erro ou a ausência de um diagnóstico fazem com que intervenções eficazes sejam improváveis de ocorrer. Isso se torna evidente quando se observam novamente os resultados obtidos no estudo PLATINO, no qual se constatou que 83,3% dos indivíduos diagnosticados com DPOC na cidade de São Paulo não recebiam qualquer tratamento farmacológico. Adicionalmente, 47,3% não foram aconselhados para a cessação do tabagismo e 72,4% não tomaram a vacina antigripal.(5)
Esses achados demonstram que a DPOC é uma doença subdiagnosticada e subtratada, podendo gerar sérias consequências para os pacientes, como maior morbidade e mortalidade, bem como acarretar um considerável impacto econômico no sistema de saúde. Logo, informações atualizadas e precisas sobre o subdiagnóstico da DPOC são importantes para auxiliar autoridades e profissionais da saúde a implantarem estratégias para a identificação dos indivíduos com a doença, a fim de ajudá-los, independentemente da sua gravidade.(6)
O objetivo do presente estudo foi determinar a taxa de subdiagnóstico nos novos casos de DPOC, após nove anos de seguimento, e compará-la à taxa de subdiagnóstico obtida nos casos prevalentes de DPOC no estudo PLATINO na fase inicial, assim como identificar as características antropométricas, clínicas e de exposição prévia a fatores de risco para a DPOC nos indivíduos subdiagnosticados na fase de seguimento do estudo PLATINO, realizado na cidade de São Paulo, em relação aos indivíduos previamente diagnosticados.
MétodosA população do presente estudo foi composta pelos mesmos indivíduos da amostra original do estudo PLATINO na fase inicial na cidade de São Paulo (n = 1.000), com a participação de um total de 613 sujeitos nessa fase de seguimento do estudo. O presente estudo contou com a aprovação do Comitê de Ética em Pesquisa da Universidade Federal de São Paulo (UNIFESP)/Hospital São Paulo (Parecer no. 04234/10), e, após serem esclarecidos sobre a pesquisa e os procedimentos, todos os indivíduos que aceitaram participar assinaram um termo de consentimento livre e esclarecido.
As informações sobre a amostragem do estudo PLATINO na fase inicial já foram descritas em um estudo prévio.(3)
Todas as casas nas quais os indivíduos foram entrevistados no estudo PLATINO na fase inicial foram visitadas. Inicialmente, o contato foi realizado por uma das quatro rastreadoras, as quais confirmaram se a pessoa ainda residia naquele endereço, conferiram o telefone e informaram sobre a visita dos entrevistadores.
Em relação aos indivíduos que não residiam mais na mesma casa (PLATINO fase inicial), as rastreadoras procuraram saber do seu paradeiro por meio de informações com vizinhos ou no comércio da vizinhança. Aqueles cujo paradeiro era ignorado tiveram seus nomes procurados no registro de óbitos dentro e fora do estado de São Paulo. Todas as informações obtidas pelas rastreadoras foram passadas à coordenadoria responsável pela organização das fichas para o posterior agendamento das entrevistas.
Previamente ao desenvolvimento da avaliação no campo, todos os entrevistadores (14 graduandos em fisioterapia e fisioterapeutas) passaram por um curso de treinamento ministrado pela equipe de São Paulo, visando à aplicação do questionário e à realização da espirometria. Certificada a coordenadoria de que os entrevistadores estavam aptos para fazer as avaliações, foi realizado um estudo piloto com o objetivo de se esclarecer dúvidas que poderiam surgir durante o trabalho de campo, encerrando assim a fase de treinamento dos entrevistadores. Concluído o estudo piloto, deu-se início, efetivamente, aos agendamentos das visitas (realizados pelos supervisores), e os entrevistados foram, em seguida, visitados por dois entrevistadores.
Na casa do entrevistado, os pesquisadores solicitavam primeiramente o termo de consentimento e, se os indivíduos concordassem em participar do estudo, dava-se seguimento à coleta de dados, obedecendo à seguinte sequência: antropometria, preenchimento do questionário sobre os critérios de exclusão para espirometria,(1) realização da espirometria pré-broncodilatador, aplicação do questionário principal (durante os 15 minutos após a aplicação do broncodilatador), realização da espirometria pós-broncodilatador e continuação da aplicação do questionário principal.
O questionário principal aplicado foi o mesmo utilizado no estudo PLATINO na fase inicial(1) (versão combinada do questionário da American Thoracic Society - Division of Lung Disease,(7) do European Community Respiratory Health Survey II,(8) e do Lung Health Study), sendo que, nessa fase de seguimento, foram acrescentadas novas questões sobre tabagismo, diagnóstico, asma, atividade física, sono e depressão.
O diagnóstico de DPOC foi confirmado por espirometria, a qual foi realizada antes e após o uso de broncodilatador, de acordo com as diretrizes da American Thoracic Society/European Respiratory Society.(9) As medições foram realizadas com um espirômetro portátil, à bateria, com um sistema de ultrassom (EasyOneT; Medical Technologies, Chelmsparad, MA, EUA e NDD Medizintechnik AG, Zurique, Suíça), idênticos aos utilizados no estudo PLATINO na fase inicial. Os indivíduos realizavam, no máximo, até 15 manobras expiratórias forçadas com o objetivo de se obter o grau de qualidade A, ou seja, três provas aceitáveis com os dois melhores valores de VEF1 e CVF, sem ultrapassar a diferença de 150 mL. A seguir, era administrado um broncodilatador (salbutamol, 200 µg) por via inalatória, utilizando-se um espaçador de 500 mL e, após 15 minutos, repetia-se a prova. Todas as provas espirométricas foram realizadas com o indivíduo sentado, usando pinça nasal e bocal descartável. Foi registrada unicamente a fase expiratória.
A gravidade da doença, com base nos dados da função pulmonar, foi classificada de acordo com os critérios do Global Initiative for Chronic Obstructive Lung Disease.(10)
Para a identificação do diagnóstico prévio de DPOC, as mesmas três questões do estudo PLATINO na fase inicial foram utilizadas: "O médico alguma vez lhe disse que o(a) Sr(a) tem enfisema nos seus pulmões?"; "O médico alguma vez lhe disse que o(a) Sr(a) tem bronquite crônica?"; "O médico alguma vez na vida lhe disse que o(a) Sr(a) tem doença pulmonar obstrutiva crônica (DPOC)?".
Foi considerado como subdiagnóstico o fato de o indivíduo responder negativamente essas três primeiras perguntas e apresentar uma relação VEF1/CVF < 0,7 na espirometria após o uso de broncodilatador.
Os dados coletados no estudo PLATINO na fase de seguimento foram adicionados à base de dados original do estudo na fase inicial, que foi realizado em 2003, em São Paulo. O programa estatístico utilizado para análise dos dados foi o Statistical Package for the Social Sciences, versão 17.0 (SPSS Inc., Chicago, IL, EUA) e o nível de significância estatística adotado foi p < 0,05.
A taxa de subdiagnóstico nos casos novos após nove anos de seguimento foi avaliada pelo teste do qui-quadrado, assim como a comparação das características dos indivíduos subdiagnosticados em relação aos previamente diagnosticados na fase de seguimento do estudo PLATINO, quando as variáveis eram categóricas. Para as variáveis numéricas, foi utilizado o teste t para amostras independentes. As características investigadas foram as seguintes: sexo, idade, escolaridade, estado nutricional, função pulmonar, gravidade da doença, sintomas, qualidade de vida e exposição aos fatores de risco relacionados à DPOC (queima de madeira/esterco ou carvão; tabagismo; e história de infecção respiratória na infância).
Para as variáveis contínuas, os resultados foram apresentados em média e desvio-padrão. As variáveis categóricas foram apresentadas em valor absoluto e em proporção, representando o número de casos dentro de cada categoria.
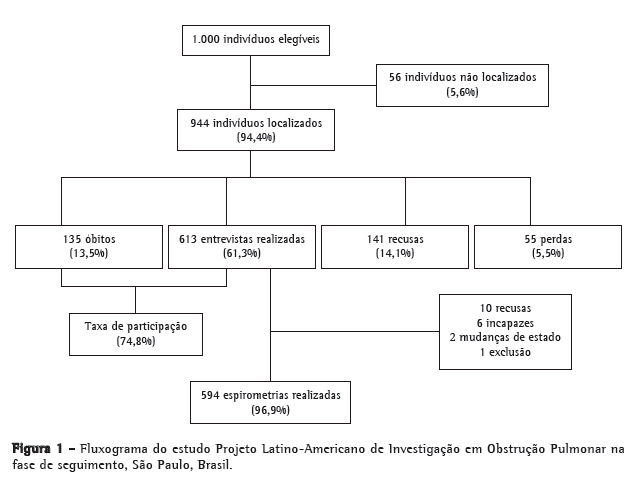
ResultadosAs informações sobre os dados dos indivíduos que participaram do estudo PLATINO na fase de seguimento na cidade de São Paulo encontram-se na Figura 1.
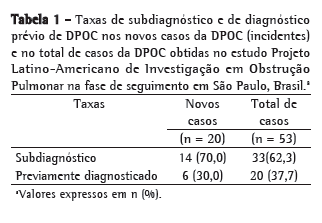
Na Tabela 1, estão demonstrados os casos de subdiagnóstico e de diagnóstico prévio de DPOC para os novos casos da doença (incidentes) e para todos os casos de DPOC na fase de seguimento do estudo PLATINO em São Paulo. Pode-se observar que 70,0% dos incidentes e 62,3% de todos os casos da DPOC nessa fase de seguimento apresentavam obstrução ao fluxo aéreo, mas não possuíam o diagnóstico médico da doença.
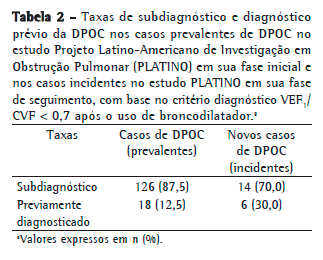
As taxas de subdiagnóstico encontradas para os casos incidentes (PLATINO fase de seguimento) e para casos prevalentes de DPOC (PLATINO fase inicial), ambos classificados pelo critério VEF1/CVF < 0,7, encontram-se descritas na Tabela 2. Houve uma redução na taxa de subdiagnóstico de 17,5%, com consequente aumento da proporção de indivíduos previamente diagnosticados com DPOC para os casos incidentes em relação aos casos prevalentes.
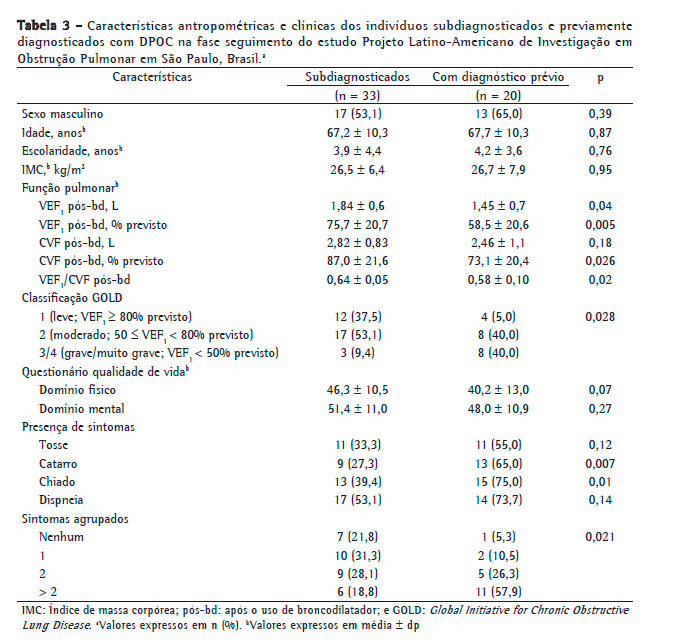
As características dos indivíduos subdiagnosticados na fase de seguimento do estudo também foram avaliadas. Encontram-se descritas as características antropométricas e clínicas (Tabela 3), assim como de exposição aos fatores de risco para DPOC (Tabela 4) dos indivíduos subdiagnosticados em relação aos que apresentavam o diagnóstico prévio da doença no estudo PLATINO na fase de seguimento. Pode-se observar que não houve diferenças estatisticamente significantes entre esses grupos em relação às características antropométricas e de exposição aos fatores de risco da doença (Tabelas 2 e 4); porém, os indivíduos subdiagnosticados apresentavam melhor função pulmonar, menor gravidade da doença, menos sintomas de catarro e chiado, assim como uma maior proporção de um ou nenhum sintoma respiratório autorrelatado.
 Discussão
DiscussãoOs resultados do estudo PLATINO na fase de seguimento realizado na cidade de São Paulo demonstram que aproximadamente dois terços dos casos novos e de todos os casos de DPOC diagnosticados após nove anos de acompanhamento não haviam recebido um diagnóstico prévio da doença, e esses indivíduos possuem um perfil clínico com presença de poucos sintomas respiratórios, melhor função pulmonar e menor gravidade da doença em relação aos previamente diagnosticados.
É previsto que a DPOC seja a terceira maior causa de morte no mundo em 2020(11) e, apesar do seu grande impacto social e econômico, essa é uma doença que ainda apresenta altas taxas de subdiagnóstico. No presente estudo, o subdiagnóstico foi de 70,0% para os novos casos de DPOC diagnosticados após nove anos de seguimento e de 62,3% ao se considerar todos os indivíduos que participaram da fase de seguimento do estudo PLATINO. Taxas similares também foram reportadas em uma amostra de unidades básicas de saúde em Aparecida de Goiânia, Brasil(12) e em estudos epidemiológicos transversais realizados em outros países, como Espanha(13) e EUA(14) (71,4%, 78,2% e 74,9%, respectivamente), demonstrando que a ausência do diagnóstico de DPOC não é uma realidade somente de países em desenvolvimento. Isso reflete que a espirometria é um exame subutilizado, à semelhança do que foi encontrado em um estudo realizado na Espanha.(15) Tal achado reforça a necessidade de maior divulgação da importância desse exame, assim como da ampliação de seu uso em unidades de atenção primária à saúde, já que a espirometria é a melhor maneira de se aumentar a detecção da DPOC,(16) o que previne o subdiagnóstico dessa doença. Adicionalmente, a adoção de medidas simples e razoáveis para a interpretação da espirometria pode auxiliar o profissional da saúde a utilizá-la para diagnosticar os indivíduos, para que esses possam ser devidamente tratados.
Comparando-se, porém, a taxa de subdiagnóstico nos novos casos de DPOC no estudo PLATINO na fase de seguimento em relação à taxa de subdiagnóstico nos casos prevalentes da DPOC no estudo PLATINO na fase inicial, houve uma redução de 17,5% (70,0% nos incidentes vs. 87,5% nos prevalentes), havendo uma duplicação na proporção de diagnósticos (30,0% nos incidentes vs. 12,5% nos prevalentes). Muito provavelmente, o maior número de diagnósticos atuais decorre da maior divulgação sobre a doença pela mídia, em função do trabalho que a sociedade médica vem fazendo. Adicionalmente, como colocamos o nosso serviço à disposição dos indivíduos que apresentaram alterações na prova de função pulmonar no estudo PLATINO na fase inicial para consulta com pneumologista, isso também pode ter sido um fator contribuinte para a redução na taxa de subdiagnóstico. No entanto, esforços ainda devem ser redobrados para que os indivíduos com DPOC sejam diagnosticados e, dessa forma, os custos e o impacto dessa doença nos seus portadores possam ser minimizados.
Os indivíduos subdiagnosticados na fase de seguimento do estudo PLATINO em São Paulo apresentavam como características a presença de poucos sintomas respiratórios, melhor função pulmonar e consequente menor gravidade da doença em relação aos previamente diagnosticados.
Estudos epidemiológicos prévios(5,6,17) e estudos em unidades de atenção primária à saúde(18,19) reportaram essas mesmas características nos indivíduos subdiagnosticados. Isso demonstra que, mesmo após nove anos de acompanhamento, os indivíduos subdiagnosticados apresentaram o mesmo perfil clínico, e o fato de serem pouco sintomáticos chama a atenção para que os profissionais da saúde passem a utilizar a espirometria para o diagnóstico. Vale ressaltar que aproximadamente 90% dos casos subdiagnosticados no presente estudo, considerando-se os novos casos da DPOC, apresentavam gravidade da doença de leve a moderada, demonstrando que a maior parte dos indivíduos subdiagnosticados está em estadiamento inicial da doença, o que torna possível uma intervenção precoce.
Como limitação do presente estudo, pode-se citar a perda de seguimento, que foi superior a 20%. Entretanto, um estudo europeu multicêntrico longitudinal e com período de seguimento similar, reportou uma taxa de participação de 63,3%.(20) Além disso, o grupo constituído por perdas e recusas apresentava as mesmas características clínicas e de função pulmonar que os indivíduos que participaram do estudo PLATINO na fase de seguimento. Outra limitação pode ser decorrente do diagnóstico de DPOC ter sido baseado no critério da Global Initiative for Chronic Obstructive Lung Disease (VEF1/CVF < 0,7), visto que esse critério pode produzir um aumento das taxas de resultados falso-positivos em indivíduos com idade mais avançada e não detectar a doença naqueles com idade mais precoce (falso-negativos)(21); por esse motivo, a utilização do limite inferior da normalidade é recomendada por alguns autores. (22,23) Foi recentemente sugerido que em casos discordantes de obstrução ao fluxo aéreo, ou seja, presença de uma relação VEF1/CVF < 0,7 e VEF1/CVF ≥ limite inferior da normalidade, tais indivíduos não apresentariam uma obstrução clinicamente significativa, porém com um perfil clínico caracterizado por importantes comorbidades, indicando que esses indivíduos poderiam estar em risco de desenvolver a doença e devendo, portanto, serem acompanhados cuidadosamente. (24) Entretanto, a determinação do melhor critério para o diagnóstico da obstrução ao fluxo aéreo ainda permanece em discussão na literatura(25,26) e ainda permanecerá, pois um recente estudo epidemiológico longitudinal(27) sugeriu a utilização do critério VEF1/VEF6, visto que a CVF varia dependendo do tempo expiratório realizado durante a manobra forçada na espirometria.
Conclui-se que a taxa de subdiagnóstico nos novos casos de DPOC identificados no estudo PLATINO na fase de seguimento em São Paulo foi de 70,0%. Houve uma redução de 17,5% na taxa de subdiagnóstico ao se comparar os casos incidentes (PLATINO fase de seguimento) em relação aos casos prevalentes (PLATINO fase inicial) e, mesmo após nove anos, os indivíduos subdiagnosticados continuam apresentando o mesmo perfil clínico (melhor função pulmonar, menor gravidade da doença e poucos sintomas).
A taxa de subdiagnóstico da DPOC identificada no presente estudo epidemiológico longitudinal de base populacional foi alta, reforçando a necessidade de maior divulgação e ampliação do uso da espirometria em unidades de atenção primária à saúde.
AgradecimentosAgradecemos à Associação Latino-Americana de Tórax (ALAT) o apoio acadêmico.
Referências1. Menezes AM, Victora CG, Perez-Padilla R; PLATINO Team. The Platino project: methodology of a multicenter prevalence survey of chronic obstructive pulmonary disease in major Latin American cities. BMC Med Res Methodol. 2004;4:15. http://dx.doi.org/10.1186/1471-2288-4-15 PMid:15202950 PMCid:PMC442126
2. Menezes AM, Jardim JR, Pérez-Padilla R, Camelier A, Rosa F, Nascimento O, Hallal PC. Prevalence of chronic obstructive pulmonary disease and associated factors: the PLATINO Study in São Paulo, Brazil. Cad Saude Publica. 2005;21(5):1565-73. http://dx.doi.org/10.1590/S0102-311X2005000500030 PMid:16158163
3. Menezes AM, Perez-Padilla R, Jardim JR, Mui-o A, Lopez MV, Valdivia G, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366(9500):1875-81. http://dx.doi.org/10.1016/S0140-6736(05)67632-5
4. Montes de Oca M, Tálamo C, Halbert RJ, Perez-Padilla R, Lopez MV, Mui-o A, et al. Health status perception and airflow obstruction in five Latin American cities: the PLATINO study. Respir Med. 2009;103(9):1376-82. http://dx.doi.org/10.1016/j.rmed.2009.03.005 PMid:19364640
5. Nascimento OA, Camelier A, Rosa FW, Menezes AM, Pérez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease is underdiagnosed and undertreated in São Paulo (Brazil): results of the PLATINO study. Braz J Med Biol Res. 2007;40(7):887-95. http://dx.doi.org/10.1590/S0100-879X2006005000133 PMid:17653440
6. Tálamo C, de Oca MM, Halbert R, Perez-Padilla R, Jardim JR, Mui-o A, et al. Diagnostic labeling of COPD in five Latin American cities. Chest. 2007;131(1):60-7. http://dx.doi.org/10.1378/chest.06-1149 PMid:17218557
7. Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118(6 Pt 2):1-120. PMid:742764
8. European Community Respiratory Health Survey II Steering Committee. The European Community Respiratory Health Survey II. Eur Respir J. 2002;20(5):1071-9. http://dx.doi.org/10.1183/09031936.02.00046802 PMid:12449157
9. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-38. http://dx.doi.org/10.1183/09031936.05.00034805 PMid:16055882
10. Global Initiative for Chronic Obstructive Lung Disease [homepage on the Internet]. Bethesda: Global Initiative for Chronic Obstructive Lung Disease. [cited 2013 Aug 20]. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Updated 2013. [Adobe Acrobat document, 99p.]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf
11. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498-504. http://dx.doi.org/10.1016/S0140-6736(96)07492-2
12. Queiroz MC, Moreira MA, Rabahi MF. Underdiagnosis of COPD at primary health care clinics in the city of Aparecida de Goiânia, Brazil. J Bras Pneumol. 2012;38(6):692-9. http://dx.doi.org/10.1590/S1806-37132012000600003 PMid:23288113
13. Pe-a VS, Miravitlles M, Gabriel R, Jiménez-Ruiz CA, Villasante C, Masa JF, et al. Geographic variations in prevalence and underdiagnosis of COPD: results of the IBERPOC multicentre epidemiological study. Chest. 2000;118(4):981-9. http://dx.doi.org/10.1378/chest.118.4.981
14. Coultas DB, Mapel D, Gagnon R, Lydick E. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med. 2001;164(3):372-7. http://dx.doi.org/10.1164/ajrccm.164.3.2004029 PMid:11500335
15. Miravitlles M, de la Roza C, Morera J, Montemayor T, Gobartt E, Martín A, et al. Chronic respiratory symptoms, spirometry and knowledge of COPD among general population. Respir Med. 2006;100(11):1973-80. http://dx.doi.org/10.1016/j.rmed.2006.02.024 PMid:16626950
16. Mannino DM. Defining chronic obstructive pulmonary disease... and the elephant in the room. Eur Respir J. 2007;30(2):189-90. http://dx.doi.org/10.1183/09031936.00058707 PMid:17666553
17. Schirnhofer L, Lamprecht B, Firlei N, Kaiser B, Buist AS, Halbert RJ, et al. Using targeted spirometry to reduce non-diagnosed chronic obstructive pulmonary disease. Respiration. 2011;81(6):476-82. http://dx.doi.org/10.1159/000320251 PMid:20720402
18. Minas M, Hatzoglou C, Karetsi E, Papaioannou AI, Tanou K, Tsaroucha R, et al. COPD prevalence and the differences between newly and previously diagnosed COPD patients in a spirometry program. Prim Care Respir J. 2010;19(4):363-70. http://dx.doi.org/10.4104/pcrj.2010.00034 PMid:20532466
19. Hill K, Goldstein RS, Guyatt GH, Blouin M, Tan WC, Davis LL, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182(7):673-8. http://dx.doi.org/10.1503/cmaj.091784 PMid:20371646 PMCid:PMC2855915
20. de Marco R, Accordini S, Marcon A, Cerveri I, Antó JM, Gislason T, et al. Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med. 2011;183(7):891-7. http://dx.doi.org/10.1164/rccm.201007-1125OC PMid:20935112
21. Celli BR, Halbert RJ, Isonaka S, Schau B. Population impact of different definitions of airway obstruction. Eur Respir J. 2003;22(2):268-73. http://dx.doi.org/10.1183/09031936.03.00075102 PMid:12952259
22. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-68. http://dx.doi.org/10.1183/09031936.05.00035205 PMid:16264058
23. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202-18. http://dx.doi.org/10.1164/ajrccm/144.5.1202 PMid:1952453
24. Lamprecht B, Schirnhofer L, Kaiser B, Buist SA, Mannino DM, Studnicka M. Subjects with Discordant Airways Obstruction: Lost between Spirometric Definitions of COPD. Pulm Med. 2011;2011:780215. doi: 10.1155/2011/780215
25. Celli BR, Halbert RJ. Point: should we abandon FEV1/FVC <0.70 to detect airway obstruction? No. Chest. 2010;138(5):1037-40. http://dx.doi.org/10.1378/chest.10-2049 PMid:21051393
26. Enright P, Brusasco V. Counterpoint: should we abandon FEV1/FVC < 0.70 to detect airway obstruction? Yes. Chest. 2010;138(5):1040-2; discussion 1042-4. http://dx.doi.org/10.1378/chest.10-2052 PMid:21051394
27. Perez-Padilla R, Wehrmeister FC, Celli BR, Lopez-Varela MV, Montes de Oca M, Mui-o A, et al. Reliability of FEV1/FEV6 to diagnose airflow obstruction compared with FEV1/FVC: the PLATINO longitudinal study. PLoS One. 2013;8(8):e67960. http://dx.doi.org/10.1371/journal.pone.0067960 PMid:23936297 PMCid:PMC3731337
*Trabalho realizado na Universidade Federal de São Paulo, São Paulo (SP) Brasil.
Endereço para correspondência: José R Jardim. Rua Botucatu, 740, 3°andar, Disciplina de Pneumologia, CEP 04023-062, São Paulo, SP, Brasil.
Fax: 55 11 5572-4301. E-mail: jardimpneumo@gmail.com
Apoio financeiro: Este estudo recebeu apoio financeiro da Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Aché Laboratórios Farmacêuticos S.A., GlaxoSmithKline e Takeda Brasil Farmacêutica.
Recebido para publicação em 20/8/2013. Aprovado, após revisão, em 22/10/2013.
Sobre os autoresGraciane Laender Moreira
Fisioterapeuta. Universidade Federal de São Paulo, São Paulo (SP) Brasil.
Beatriz Martins Manzano
Fisioterapeuta. Universidade Federal de São Paulo, São Paulo (SP) Brasil.
Mariana Rodrigues Gazzotti
Professora Doutora. Departamento de Fisioterapia, Centro Universitário São
Camilo, São Paulo (SP) Brasil.
Oliver Augusto Nascimento
Médico Assistente. Disciplina de Pneumologia, Universidade Federal de São Paulo, São Paulo (SP) Brasil.
Rogelio Perez-Padilla
Professor. Instituto Nacional de Enfermedades Respiratórias, Cidade do México, México.
Ana Maria Baptista Menezes
Professora Titular de Pneumologia. Departamento de Clínica Médica, Faculdade de Medicina, Universidade Federal de Pelotas, Pelotas (RS) Brasil.
José Roberto Jardim
Professor Livre-Docente. Disciplina de Pneumologia, Universidade Federal de São Paulo, São Paulo (SP) Brasil.






