ABSTRACT
Objective: To cross-culturally adapt the Wisconsin Smoking Withdrawal Scale (WSWS) for use in Brazil and evaluate the reproducibility of the new (Brazilian Portuguese-language) version. Methods: The original English version of the WSWS was translated into Brazilian Portuguese. For cross-cultural adaptation, the Brazilian Portuguese-language version of the WSWS was administered to eight volunteers, all of whom were smokers. After adjustments had been made, the WSWS version was back-translated into English. The Brazilian Portuguese-language version was thereby found to be accurate. The final Brazilian Portuguese-language version of the WSWS was applied to 75 smokers at three distinct times. For the assessment of interobserver reproducibility, it was applied twice within a 30-min interval by two different interviewers. For the assessment of intraobserver reproducibility, it was applied again 15 days later by one of the interviewers. Intraclass correlation coefficients (ICCs) were used in order to test the concordance of the answers. The significance level was set at p < 0.05. Results: Of the 75 volunteers, 43 (57.3%) were female. The overall mean age was 46.3 years. Interobserver and intraobserver reproducibility was determined for each of the WSWS seven domains, the ICCs for which ranged from 0.87 to 0.94 and from 0.76 to 0.92, respectively. The mean time to completion of the WSWS was 6 min and 44 s, and the response time per question ranged from 4.2 to 12.6 s. Conclusions: The Brazilian Portuguese-language version of the WSWS is reproducible, fast, and simple. It can therefore be used as a tool for assessing the severity of the symptoms of nicotine withdrawal syndrome.
Keywords:
Nicotine; Tobacco use disorder; Substance withdrawal syndrome; Reproducibility of results.
RESUMO
Objetivo: Adaptar culturalmente e avaliar a reprodutibilidade da Wisconsin Smoking Withdrawal Scale (WSWS) para o português do Brasil. Métodos: Foi realizada a tradução da versão original em língua inglesa para o português. A versão traduzida foi aplicada em 8 voluntários fumantes para a adaptação cultural. Após ajustes, a versão da WSWS foi submetida à tradução retrógrada do português para o inglês. A versão em português do Brasil foi considerada adequada. Para a avaliação da reprodutibilidade, a escala foi aplicada em 75 fumantes em dois momentos, com intervalo de 30 minutos (reprodutibilidade interobservador) e, num terceiro momento, após 15 dias (reprodutibilidade intraobservador). Utilizou-se o coeficiente de correlação intraclasse (CCI) para testar a concordância entre as respostas. O nível de significância adotado foi p < 0,05. Resultados: Dos 75 voluntários, 43 (57,3%) eram do gênero feminino. A média geral de idade foi 46,3 anos. A reprodutibilidade interobservador e intraobservador foi determinada para cada um dos sete domínios da WSWS, com CCI variando de, respectivamente, 0,87 a 0,94 e de 0,76 a 0,92. O tempo médio de resposta da WSWS foi 6 min e 44 s, e o tempo de resposta para cada questão variou de 4,2 a 12,6 s. Conclusões: A versão da WSWS para o português do Brasil é reprodutível, de aplicação rápida e simples, podendo ser utilizada como um instrumento de avaliação da gravidade dos sintomas da síndrome da abstinência à nicotina.
Palavras-chave:
Nicotina; Transtorno por uso de tabaco; Síndrome de abstinência a substâncias; Reprodutibilidade dos testes.
IntroductionIn Brazil, 900,000 deaths occur each year. Of those deaths, 200,000 are from smoking. According to the World Health Organization, 16% of all new cancer cases and 40% of all cancer deaths are due to smoking, as are 20% of all cases of cardiovascular disease and 75% of all cases of nonmalignant respiratory disease, 25% of all smokers dying from smoking-related diseases. Smoking is therefore the leading preventable cause of death worldwide. In Brazil, there are approximately 24 million smokers, who account for 17% of the Brazilian population.(1,2)
Cigarette smoke contains more than 4,700 compounds. Of those, nicotine is the only one that is known to cause dependence, by binding to cholinergic receptors in the midbrain, rapidly leading to tolerance and addiction. The neurobiological changes caused by nicotine are intense, and the intensity of smoking can alternately trigger feelings of pleasure and depression.(3,4)
In nicotine-dependent smokers, smoking cessation can lead to nicotine withdrawal syndrome, which is described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) as an intense craving for nicotine accompanied by difficulty concentrating, mental confusion, depression, irritability, anxiety, cardiac rhythm disturbances, sleep disturbances, asthenia, and increased appetite with weight gain. These symptoms can have an early onset (60 min after smoking cessation) and last several days (10-15 days).(5-7)
Among the smoking cessation strategies that are currently available, the combination of cognitive-behavioral therapy and nicotine replacement therapy with prescription-free transdermal nicotine patches has shown good results in various countries. Groups of up to 10 smokers meet with the objective of sharing their experiences and helping one another during the smoking cessation period.(8,9)
There have been numerous experimental studies involving the use of specific questionnaires and scales. The main objective is to use an instrument that is useful for answering the study question by providing qualitative and quantitative information. In addition to being noninvasive, inexpensive, and easy to use, questionnaires and scales will be adequately reproducible in specific cultures if they have good internal consistency and accuracy.(10,11)
Various questionnaires and scales have been developed in order to assess nicotine withdrawal symptoms and improve quality of life.(10-13) In Minnesota, USA, Hughes et al. developed and validated a specific scale to evaluate the effects of smoking abstinence; the scale was designated Wisconsin Smoking Withdrawal Scale (WSWS).(11) A higher WSWS score translates to a higher level of nicotine dependence, as evidenced by the symptoms of nicotine withdrawal.(11) The hypothesis is that the WSWS can identify individuals who are prone to relapse on the basis of the symptoms of nicotine withdrawal. In Brazil, there is no scale evaluating the effects of smoking abstinence on individuals undergoing smoking cessation treatment. Therefore, it is of utmost importance to translate and culturally adapt the WSWS, given that the scale can aid therapists in predicting relapse on the basis of nicotine withdrawal symptoms. The objective of the present study was to translate and cross-culturally adapt the WSWS for use in Brazil, as well as to evaluate the reproducibility of the new (Brazilian Portuguese-language) version.
MethodsWe conducted a prospective cohort study to culturally adapt the WSWS for use in Brazil and evaluate the reproducibility of the new (Brazilian Portuguese-language) version. The study project was approved by the Research Ethics Committee of the Universidade Estadual de Ciências da Saúde de Alagoas (UNCISAL, Alagoas State University of Health Sciences; Protocol no. 808), located in the city of Maceió, Brazil, where the data were collected. All of the participants gave written informed consent, and the study was conducted in accordance with Brazilian National Health Council Resolution 196/96.
The WSWS was developed by a group of researchers led by Welsch et al. and validated for use in English in 1999.(11) The WSWS is a 28-item scale divided into seven domains: anger; anxiety; concentration; craving; hunger; sadness; and sleep. The scores for each domain range from 0 to 4 (maximum total score, 112), a higher score translating to a higher prevalence of nicotine withdrawal syndrome and a greater likelihood of relapse.(11)
The questions are arranged by domain rather than in numerical order. The WSWS score is progressive, i.e., a higher final score translates to a higher number of nicotine withdrawal symptoms. Questions 1, 2, 4, 10, 17, 22, and 24 are reverse-scored items, i.e., a higher score translates to a less severe withdrawal syndrome. This was relevant to the preparation of the data for statistical analysis, the scores for the abovementioned questions having been reversed before the analysis.
The original English-language version of the WSWS was translated to Brazilian Portuguese by a Brazilian translator who was proficient in English. The translated version of the scale was back-translated to English by a translator who was a native speaker of English and who was proficient in Portuguese. Neither translator knew the original WSWS.
For cross-cultural adaptation, an expert committee comprising a specialist in nicotine withdrawal syndrome (proficient in English and in Portuguese), the authors of the present study, and the author of the original scale convened in order to discuss how to adapt the WSWS for use in Brazil without changing the essence of the scale.
The Brazilian Portuguese-language version of the WSWS was administered to 8 volunteers, all of whom were smokers, in order to determine whether the questions were easy to understand and whether there were any questions regarding the text. The final version of the Brazilian Portuguese-language WSWS was arrived at after its cross-cultural adaptation and submission to the author of the original scale.
In order to evaluate the reproducibility of the Brazilian-Portuguese language version of the WSWS, the scale was administered to a convenience sample of 75 volunteers, all of whom were smokers, in accordance with a study by Hopkins.(12) The volunteers were selected from among patients being treated at the UNCISAL Núcleo de Apoio à Prevenção e Cessação do Tabagismo (PREVFUMO, Center for Support on Smoking Prevention and Cessation) Outpatient Clinic, where they were evaluated. Our sample size was adequate, given that previous studies cross-culturally adapting and evaluating the reproducibility of respiratory disease questionnaires for use in Brazil, including the Saint George's Respiratory Questionnaire(13) and Airway Questionnaire 20,(14) evaluated 30 patients each.
The inclusion criteria were as follows: being over 18 years of age; having decided to quit smoking; and showing an appropriate cognitive level, as assessed by the Mini-Mental State Examination (MMSE).(15) The exclusion criteria were as follows: having had acute myocardial infarction or stroke in the three months preceding the evaluation; having uncontrolled chronic disease; having malignant disease; having disabling disease; being pregnant; having an MMSE score < 21; and showing exhaled carbon monoxide (eCO) levels 6 ppm.(8)
Initially, we administered the MMSE questionnaire to the volunteers in order to evaluate their mental state (cognitive level). Subsequently, we collected demographic data and administered the Brazilian Economic Classification Criterion questionnaire.
On evaluation day 1 (T0) at the PREVFUMO Outpatient Clinic, the volunteers completed the following: the Brazilian Portuguese-language version of the WSWS; the Fagerström Test for Nicotine Dependence (FTND), previously adapted for use in Brazil(16); and the hospital anxiety and depression scale (HADS), previously validated for use in Brazil.(17) In addition, eCO levels were measured.(18,19) On the same day, the WSWS was administered to the volunteers for the second time, 30 min after T0 (T1), by a different investigator, in order to assess interobserver reproducibility. Fifteen days after T0, before the initiation of smoking cessation treatment, the Brazilian-Portuguese language version of the WSWS was administered to the volunteers for the third time (T2), by the same investigator who had administered it at T0, in order to assess intraobserver reproducibility. In order to minimize symptoms of anxiety and depression, we did not tell the volunteers when their smoking cessation treatment was going to start.
Prior to each evaluation, we measured eCO levels using an eCO monitor (SmokeCheck®; MicroDirect Inc., Lewiston, ME, USA) in order to determine whether the volunteers were indeed smokers (eCO levels > 6 ppm)(8) and whether they were light smokers (eCO levels = 7-10 ppm), moderate smokers (eCO levels = 11-20 ppm), or heavy smokers (eCO levels > 20 ppm).(17) In order to measure eCO levels, we asked the volunteers to hold their breath for 20 s. This allowed a better balance between blood carbon monoxide levels and alveolar carbon monoxide levels, therefore improving measurement accuracy. After the inspiratory pause, the volunteers were instructed to exhale slowly and completely with their lips around the mouthpiece of the device in order to prevent air leakage.(19)
In the statistical analysis, categorical variables were expressed as absolute and relative frequencies (proportions), whereas continuous variables were expressed as means, standard deviations, medians, and overall range (minimum and maximum). We used the chi-square test in order to determine the correlation between two variables with normal distribution. We used the Student's t-test in order to compare two independent variables with normal distribution. We used the Mann-Whitney test in order to compare two independent variables with non-normal distribution.(20)
We used the intraclass correlation coefficient (ICC) in order to assess the reproducibility of the WSWS. The ICC ranges from 0 to 1, ICC values closer to 1 translating to a greater reproducibility of the variable. In order to measure the level of agreement between two assessments sorted into categories (WSWS questions), we used the kappa reliability coefficient. Finally, we used nonparametric Spearman's correlation coefficient in order to determine the correlation between two minimally ordinal variables, taking into consideration the distributions of the variables studied or the variability of the measurements taken.(21)
For all statistical tests, the level of significance was set at a type I error < 0.05 or 5%. The statistical analysis was performed with the Statistical Package for the Social Sciences, version 13.0 (SPSS Inc., Chicago, IL, USA).
ResultsAfter the WSWS was first translated to Brazilian Portuguese, we evaluated 8 volunteers, 6 of whom were male. The mean age was 38.0 ± 11.2 years. The Brazilian Portuguese-language version of the WSWS was well understood, no questions having arisen. The only modification was that all items beginning with "sinto-me" were changed to "eu tenho sentido." This change was tested in another 30 volunteers, and "eu tenho sentido" was chosen over "sinto-me". The time to complete the WSWS remained unchanged, as did patient understanding of the scale. The items beginning with "sinto-me" were changed to "eu tenho sentido" because the latter was found to be more in keeping with Brazilian Portuguese than was the former. The time to complete the WSWS was recorded by two investigators and was found to be 6 min and 44 s. The response time per question ranged from 4.2 s to 12.6 s (SD = 4.18 s).
In order to assess the reproducibility of the Brazilian Portuguese-language version of the WSWS, we selected 76 volunteers (smokers only) from among those being treated at the PREVFUMO Outpatient Clinic. Of the 76 smokers, only 1 had an MMSE score < 21 and was therefore excluded from the evaluation. Of the 75 volunteers evaluated, most were female, had completed at least high school, and belonged to socioeconomic class A or B (Table 1).

The mean age of the volunteers was 46.3 ± 10.5 years. Although the body mass index characterized the study population as overweight, the values were very close to the normal range. The mean smoking history was 28.5 ± 18.5 pack-years, nicotine dependence being low (Table 2).

There were no statistically significant differences between the genders in terms of the level of education (p = 0.17) or the socioeconomic class (p = 0.79), as assessed by the chi-square test. On the basis of the levels of eCO, 65.3% of the volunteers were classified as heavy smokers (eCO levels > 20 ppm).
There were no significant differences between the genders in terms of the level of nicotine dependence (p = 0.60) or the smoking history (p = 0.57), as assessed by the Mann-Whitney test. Likewise, the HADS scores (for anxiety and depression) did not correlate with the FTND scores, as assessed by Spearman's correlation coefficient.
Table 3 shows the means and standard deviations, as well as the overall range (minimum and maximum), for each WSWS domain at T0 and T1, the scale having been administered by different investigators (interobserver reproducibility). The ICC values were higher than 0.75, and the 95% CI ranged from 0.80 to 0.93, intraclass correlation being therefore excellent.

Table 4 shows the means, ICCs, and 95% CIs for each WSWS domain at T0 and T2, the scale having been administered by the same investigator (intraobserver reproducibility). All ICC values were higher than 0.75, intraobserver reproducibility being therefore excellent. However, the lower limits of the 95% CI were lower than 0.75 for the anger, anxiety, concentration, and sadness domains.

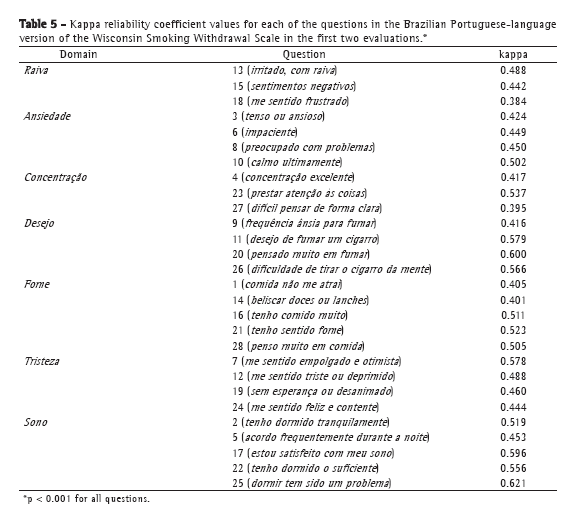
The kappa reliability coefficient was used in order to assess the reproducibility of each WSWS question. Most questions showed moderate to high reproducibility, questions 18 and 27 being the only questions showing a kappa reliability coefficient of less than 0.4 (Table 5).
 Discussion
DiscussionThe Brazilian Portuguese-language version of the WSWS showed excellent reproducibility, with ICC values greater than 0.76 for all domains, demonstrating excellent agreement between the answers. The different observers had no influence on the answers given by the volunteers, as evidenced by the similar scores obtained at T0, T1, and T2. In addition, the kappa reliability coefficient showed excellent results, with values above 0.4 for 92.9% of the WSWS questions.(21)
The WSWS can be self-administered, and the volunteers had no difficulty understanding or answering the questions. The volunteers had MMSE scores higher than 21, and most belonged to socioeconomic class A or B. This possibly had a direct influence on their understanding of the questions. We analyzed the reproducibility of the WSWS before the initiation of smoking cessation treatment in order to prevent withdrawal-related factors from affecting the reproducibility of the scale.(22) The investigators read and partly explained the questionnaire to those volunteers who had difficulty reading.
We found no correlation between the smoking history and the FTND scores. This finding is consistent with those of other studies in the literature, in which the correlation between smoking history and FTND scores was found to be poor, a finding that shows that there is no direct association between the number of cigarettes smoked per year and the level of nicotine dependence.(21) Other studies, however, have shown a strong correlation between the abovementioned variables; this shows that, despite being controversial, such findings are relevant for future studies.(5,16) It should be taken into consideration that self-reported smoking history might not be reliable, given that many smokers underestimate the number of cigarettes smoked, such underestimation being deliberate or due to a recall bias.(23)
We found a poor correlation between the levels of eCO and the FTND scores. Although the study participants showed, on average, a low level of nicotine dependence, most showed eCO levels that were consistent with heavy smoking. These findings are consistent with those of various studies in which volunteers were found to have a low level of nicotine dependence (median, 5) and high (> 20 ppm) eCO levels, a finding indicating a weak correlation between those variables and underscoring the difficulty in quantifying nicotine dependence.(23-25) We believe that the anxiety generated by the imminent entry into the smoking cessation program led the volunteers to smoke more than usual in the hours preceding the interview, and this raised the levels of eCO.
Studies in the literature have shown an increase in negative affects in smokers,(26,27) as well as high levels of anxiety and depression in those who are about to enter a smoking cessation program.(28,29) The fact that our facility is a referral center for smoking cessation might have influenced the smokers evaluated in the present study to seek treatment there, those individuals having high levels of anxiety and depression, which were primarily due to failed attempts to quit smoking.
We found no significant correlation between the HADS scores and the level of nicotine dependence. This finding is consistent with those of studies showing no correlation between a trend toward depression (as evidenced by HADS scores) and nicotine dependence (as assessed by FTND scores).(28,29) However, studies have shown a significant positive correlation of anxiety and depression with nicotine dependence, a finding that is consistent with the DSM-IV.(5,30) The conflicting results might be due to the different instruments employed, as shown in a study that found poor agreement between FTND scores and the DSM-IV. The authors of that study stated that the DSM-IV defines nicotine dependence in terms of psychiatric symptoms, whereas the FTND gives greater weight to the pleasure of smoking. Therefore, the measurement of nicotine dependence should be multifactorial, meaning that nicotine dependence cannot be measured by a single instrument.(30)
In conclusion, the present study showed that it is possible to translate and culturally adapt an instrument originally developed in a foreign language, the translated version showing excellent reproducibility. All of the domains of the Brazilian Portuguese-language version of the WSWS showed excellent intraobserver and interobserver reproducibility. The Brazilian Portuguese-language version of the WSWS is reproducible and easy to use. It can therefore be used as a tool for assessing the severity of the symptoms of nicotine withdrawal in the Brazilian population.
References1. Guriérrez AJ. El tabaquismo como problema de salud pública. In: Ferrero MB, Mezquita MA, García MT, editors. Manual de prevención y tratamiento del tabaquismo. 3rd ed. Madrid: Ergon; 2003. p. 27-68.
2. Campo-Arias A. The prevalence of nicotine-dependency in some populations: a systematic review [Article in Spanish]. Rev Salud Publica (Bogota). 2006;8(1):98-107. http://dx.doi.org/10.1590/S0124-00642006000100009
3. Hutton JJ, Hackney C. Metabolism of cigarette smoke condensates by human and rat homogenates to form mutagens detectable by Salmonella typhimurium TA1538. Cancer Res. 1975;35(9):2461-8. PMid:1097108.
4. Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly
decreases the activity of brain reward systems. J Neurosci. 2005;25(26):6208-12. PMid:15987950. http://dx.doi.org/10.1523/JNEUROSCI.4785-04.2005
5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994.
6. Picciolo M, Gigante D, Nunziata A. Nicotine addiction and current therapy of smoking cessation [Article in Italian]. Clin Ter. 2005;156(4):159-71. PMid:16342517.
7. Miranda M, Slachevsky A, Venegas P. Delirium from nicotine withdrawal in a post-operative adult patient [Article in Spanish]. Rev Med Chil. 2005;133(3):385-6. PMid:15880196.
8. Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers' gender. Exp Clin Psychopharmacol. 2006;14(2):121 35. PMid:16756416 PMCid:1564049. http://dx.doi.org/10.1037/1064-1297.14.2.121
9. Otero UB, Perez Cde A, Szklo M, Esteves GA, dePinho MM, Szklo AS, et al. Randomized clinical trial: effectiveness of the cognitive-behavioral approach and the use of nicotine replacement transdermal patches for smoking cessation among adults in Rio de Janeiro, Brazil [Article in Portuguese]. Cad Saude Publica. 2006;22(2):439 49. PMid:16501756. http://dx.doi.org/10.1590/S0102-311X2006000200021
10. Etter JF, Le Houezec J, Perneger TV. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology. 2003;28(2):359-70. PMid:12589389. http://dx.doi.org/10.1038/sj.npp.1300030
11. Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354-61. PMid:10609970. http://dx.doi.org/10.1037/1064-1297.7.4.354
12. Hopkins WG. Measures of reliability in sports medicine and science. Sports Med. 2000;30(1):1-15. PMid:10907753. http://dx.doi.org/10.2165/00007256-200030010-00001
13. Souza TC, Jardim JR, Jones P. Validação do Questionário do Hospital Saint George na Doença Respiratória (SGRQ) em pacientes portadores de doença pulmonar obstrutiva crônica no Brasil. J Pneumol. 2000;26(3):119-28. http://dx.doi.org/10.1590/S0102-35862000000300004
14. Camelier A, Rosa FW, Jones PW, Jardim JR. Brazilian version of airways questionnaire 20: a reproducibility study and correlations in patients with COPD. Respir Med. 2005;99(5):602-8. PMid:15823458. http://dx.doi.org/10.1016/j.rmed.2004.09.022
15. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189 98. http://dx.doi.org/10.1016/0022-3956(75)90026-6
16. Carmo JT, Pueyo AA. A adaptação do português do Fagerström Test for Nicotine Dependence (FTND) para avaliar a dependência e tolerância à nicotina em fumantes brasileiros. RBM Rev Bras Med. 2002;59(1/2):73-80.
17. Botega NJ, Bio MR, Zomignani MA, Garcia C Jr, Pereira WA. Mood disorders among inpatients in ambulatory and validation of the anxiety and depression scale HAD [Article in Portuguese].Rev Saude Publica. 1995;29(5):355-63. PMid:8731275.
18. Santos UP, Gannam S, Abe JM, Esteves PB, Filho MF, Wakassa TB, et al. Emprego da determinação de monóxido de carbono no ar exalado para a detecção do consumo de tabaco. J Pneumol. 2001;27(5):231-236. http://dx.doi.org/10.1590/S0102-35862001000500001
19. Reichert J, Araújo AJ, Gonçalves CM, Godoy I, Chatkin JM, Sales MP, et al. Smoking cessation guidelines--2008. J Bras Pneumol. 2008;34(10):845-80. Erratum in: J Bras Pneumol. 2008;34(12):1090. PMid:19009219. http://dx.doi.org/10.1590/S1806-37132008001000014
20. Siegel S. Estatística não-paramétrica para ciências do comportamento. São Paulo: McGraw Hill; 1981.
21. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-8. http://dx.doi.org/10.1037/0033-2909.86.2.420
22. West R, Ussher M, Evans M, Rashid M. Assessing DSM-IV nicotine withdrawal symptoms: a comparison and evaluation of five different scales. Psychopharmacology (Berl). 2006;184(3-4):619-27. PMid:16308727. http://dx.doi.org/10.1007/s00213-005-0216-z
23. Burling AS, Burling TA. A comparison of self-report measures of nicotine dependence among male drug/alcohol-dependent cigarette smokers. Nicotine Tob Res. 2003;5(5):625-33. http://dx.doi.org/10.1080/1462220031000158708
24. Meneses-Gaya IC, Zuardi AW, Loureiro SR, Crippa JA. Psychometric properties of the Fagerström Test for Nicotine Dependence. J Bras Pneumol. 2009;35(1):73 82. PMid:19219334. http://dx.doi.org/10.1590/S1806-37132009000100011
25. Brown C, Madden PA, Palenchar DR, Cooper-Patrick L. The association between depressive symptoms and cigarette smoking in an urban primary care sample. Int J Psychiatry Med. 2000;30(1):15-26. PMid:10900558. http://dx.doi.org/10.2190/NY79-CJ0H-VBAY-5M1U
26. Karp I, O'Loughlin J, Hanley J, Tyndale RF, Paradis G. Risk factors for tobacco dependence in adolescent smokers. Tob Control. 2006;15(3):199-204. PMid:16728750 PMCid:2564659. http://dx.doi.org/10.1136/tc.2005.014118
27. Cosci F, Schruers KR, Pistelli F, Griez EJ. Negative affectivity in smokers applying to smoking cessation clinics: a case-control study. Depress Anxiety. 2009;26(9):824-30. PMid:19105219. http://dx.doi.org/10.1002/da.20473
28. Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. Am J Public Health. 2000;90(7):1122-7. http://dx.doi.org/10.2105/AJPH.90.7.1122
29. Psujek JK, Martz DM, Curtin L, Michael KD, Aeschleman SR. Gender differences in the association among nicotine dependence, body image, depression, and anxiety within a college population. Addict Behav. 2004;29(2):375 80. PMid:14732426. http://dx.doi.org/10.1016/j.addbeh.2003.08.031
30. Spada MM, Nikcević AV, Moneta GB, Wells A. Metacognition as a mediator of the relationship between emotion and smoking dependence. Addict Behav. 2007;32(10):2120-9. PMid:17307299. http://dx.doi.org/10.1016/j.addbeh.2007.01.012
* Study carried out at the Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM, Federal University of São Paulo/Paulista School of Medicine - São Paulo, Brazil.
Correspondence to: Ilka L. Santoro. Disciplina de Pneumologia, Rua Botucatu, 740, 3º andar, CEP 04023-062, São Paulo, SP, Brasil.
Tel. 55 11 5576-4238. Email: ilka@pneumo.epm.br
Financial support: None.
Submitted: 11 April 2012. Accepted, after review: 20 September 2012.
About the authorsBoanerges Lopes de Oliveira Junior
Attending Physiotherapist. Universidade Estadual de Ciências da Saúde de Alagoas - UNCISAL, Alagoas State University of Health Sciences - Maceió, Brazil.
José Roberto Jardim
Tenured Adjunct Professor. Department of Pulmonology, Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM, Federal University of São Paulo/Paulista School of Medicine - São Paulo, Brazil.
Oliver Augusto Nascimento
Attending Physician. Department of Pulmonology, Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM, Federal University of São Paulo/Paulista School of Medicine - São Paulo, Brazil.
George Márcio da Costa e Souza
Assistant Professor. Department of Physical Therapy, Universidade Estadual de Ciências da Saúde de Alagoas - UNCISAL,
Alagoas State University of Health Sciences - Maceió, Brazil.
Timothy B. Baker
Director of Research. Center for Tobacco Research and Intervention, University of Wisconsin, Madison, WI, USA.
Ilka Lopes Santoro
Affiliate Professor. Department of Pulmonology, Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM, Federal University of São Paulo/Paulista School of Medicine - São Paulo, Brazil.








