ABSTRACT
Objective: To cross-culturally adapt the Wisconsin Smoking Withdrawal Scale (WSWS) for use in Brazil and evaluate the reproducibility of the new (Brazilian Portuguese-language) version. Methods: The original English version of the WSWS was translated into Brazilian Portuguese. For cross-cultural adaptation, the Brazilian Portuguese-language version of the WSWS was administered to eight volunteers, all of whom were smokers. After adjustments had been made, the WSWS version was back-translated into English. The Brazilian Portuguese-language version was thereby found to be accurate. The final Brazilian Portuguese-language version of the WSWS was applied to 75 smokers at three distinct times. For the assessment of interobserver reproducibility, it was applied twice within a 30-min interval by two different interviewers. For the assessment of intraobserver reproducibility, it was applied again 15 days later by one of the interviewers. Intraclass correlation coefficients (ICCs) were used in order to test the concordance of the answers. The significance level was set at p < 0.05. Results: Of the 75 volunteers, 43 (57.3%) were female. The overall mean age was 46.3 years. Interobserver and intraobserver reproducibility was determined for each of the WSWS seven domains, the ICCs for which ranged from 0.87 to 0.94 and from 0.76 to 0.92, respectively. The mean time to completion of the WSWS was 6 min and 44 s, and the response time per question ranged from 4.2 to 12.6 s. Conclusions: The Brazilian Portuguese-language version of the WSWS is reproducible, fast, and simple. It can therefore be used as a tool for assessing the severity of the symptoms of nicotine withdrawal syndrome.
Keywords:
Nicotine; Tobacco use disorder; Substance withdrawal syndrome; Reproducibility of results.
RESUMO
Objetivo: Adaptar culturalmente e avaliar a reprodutibilidade da Wisconsin Smoking Withdrawal Scale (WSWS) para o português do Brasil. Métodos: Foi realizada a tradução da versão original em língua inglesa para o português. A versão traduzida foi aplicada em 8 voluntários fumantes para a adaptação cultural. Após ajustes, a versão da WSWS foi submetida à tradução retrógrada do português para o inglês. A versão em português do Brasil foi considerada adequada. Para a avaliação da reprodutibilidade, a escala foi aplicada em 75 fumantes em dois momentos, com intervalo de 30 minutos (reprodutibilidade interobservador) e, num terceiro momento, após 15 dias (reprodutibilidade intraobservador). Utilizou-se o coeficiente de correlação intraclasse (CCI) para testar a concordância entre as respostas. O nível de significância adotado foi p < 0,05. Resultados: Dos 75 voluntários, 43 (57,3%) eram do gênero feminino. A média geral de idade foi 46,3 anos. A reprodutibilidade interobservador e intraobservador foi determinada para cada um dos sete domínios da WSWS, com CCI variando de, respectivamente, 0,87 a 0,94 e de 0,76 a 0,92. O tempo médio de resposta da WSWS foi 6 min e 44 s, e o tempo de resposta para cada questão variou de 4,2 a 12,6 s. Conclusões: A versão da WSWS para o português do Brasil é reprodutível, de aplicação rápida e simples, podendo ser utilizada como um instrumento de avaliação da gravidade dos sintomas da síndrome da abstinência à nicotina.
Palavras-chave:
Nicotina; Transtorno por uso de tabaco; Síndrome de abstinência a substâncias; Reprodutibilidade dos testes.
IntroduçãoAnualmente, 900 mil pessoas morrem no Brasil por diversas causas, sendo 200 mil dessas mortes decorrentes do tabagismo. Segundo a Organização Mundial de Saúde, 16% e 40% de novos casos e mortes por câncer, respectivamente, são atribuídos ao tabagismo, além de 20% dos casos de doenças cardiovasculares e de 75% dos casos de doenças respiratórias não malignas, levando à morte 25% dos fumantes por doenças relacionadas ao consumo de tabaco. Dessa forma, o tabagismo é considerado a maior causa evitável de morte no mundo. No Brasil, 17% da população ainda são fumantes (aproximadamente 24 milhões de pessoas).(1,2)
Dos mais de 4.700 componentes encontrados no cigarro, a nicotina é o único conhecido que traz dependência ao usuário, por se ligar aos receptores colinérgicos mesencefálicos, induzindo rapidamente a um processo de tolerância e dependência química. A modificação nicotínica neurobiológica é intensa e, com a intensidade do uso do tabaco, pode desencadear sensações prazerosas e depressivas de forma alternada.(3,4)
Uma vez instalada a dependência, a anulação do consumo pode levar à síndrome da abstinência, descrita e caracterizada no Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) por um forte desejo de voltar a fumar, dificuldade de concentração, confusão mental, depressão, irritabilidade, ansiedade, alteração no sono e no ritmo cardíaco; astenia e aumento do apetite com ganho ponderal. Essas manifestações podem ser precoces (60 min após a cessação) e durar por vários dias (10-15 dias).(5-7)
Atualmente, tem se buscado estratégias para auxiliar os fumantes a abandonar a dependência à nicotina, e o método de abordagem cognitivo-comportamental, associado à reposição de nicotina por meio de adesivos transdérmicos de livre prescrição, tem demonstrado bons resultados em diversos países. As reuniões são feitas em grupos de até 10 fumantes com o objetivo de encorajar o compartilhamento das experiências durante o período da cessação e a ajuda mútua.(8,9)
O modelo de pesquisa experimental que utiliza questionários e escalas específicos tem sido amplamente utilizado no universo científico. O objetivo principal é usar um instrumento que seja útil para a resposta da pergunta de pesquisa, extraindo informações qualitativas e quantitativas. Trata-se de um método não invasivo, de baixo custo e de fácil aplicação que, se apresentar boa consistência interna e acurácia, provavelmente possuirá adequada reprodutibilidade em culturas específicas.(10,11)
Para avaliar os sintomas da síndrome da abstinência e melhorar a qualidade de vida, diversos questionários e escalas têm sido desenvolvidos.(10-13) Em Minnesota, EUA, Hughes et al. desenvolveram uma escala específica para avaliar os efeitos da abstinência que foi readaptada e validada com o nome de Wisconsin Smoking Withdrawal Scale (WSWS).(11) Nessa escala, quanto maior for a pontuação do fumante, maior é seu grau de dependência nicotínica, em função da presença dos sintomas da síndrome da abstinência.(11) A hipótese é que a aplicação dessa escala possa predizer possíveis indivíduos predispostos a recaídas, de acordo com a presença dos sintomas de abstinência. No Brasil, não há uma escala que avalie os efeitos da abstinência na cessação do tabagismo. Dessa forma, é de fundamental importância a tradução e a adaptação cultural dessa escala, pois a mesma, ao avaliar os sintomas da abstinência, poderia ajudar os terapeutas a avaliar com antecedência a possibilidade de recaída. Portanto, o presente estudo teve como objetivo traduzir, adaptar culturalmente e testar a reprodutibilidade de uma versão da WSWS para uso no Brasil.
MétodosRealizamos um estudo de coorte prospectivo para adaptar culturalmente e avaliar a reprodutibilidade da escala WSWS. O projeto de pesquisa foi aprovado, sob o nº 808, pelo Comitê de Ética da Universidade Estadual de Ciências da Saúde do Estado de Alagoas (UNCISAL), localizada na cidade de Maceió, onde foram coletados os dados. Todos os participantes assinaram o termo de consentimento livre e esclarecido, conforme a resolução 196/96 do Conselho Nacional de Saúde.
A WSWS foi desenvolvida por pesquisadores liderados por Welsch et al., e validada para sua língua de origem em 1999.(11) Essa escala possui 28 itens, divididos em sete domínios: raiva, ansiedade, concentração, desejo intenso, fome, tristeza e sono. Cada domínio possui pontuação entre 0 e 4 pontos, sendo que quanto maior o escore (máximo de 112), maior é a prevalência dos sintomas da síndrome da abstinência e maior é a possibilidade de recaídas.(11)
As questões não são organizadas em ordem numérica, e sim, por domínios. A pontuação da escala é progressiva, ou seja, quanto maior for o escore final, mais sintomas de abstinência à nicotina tem o indivíduo. As questões 1, 2, 4, 10, 17, 22 e 24 possuem pontuação reversa, ou seja, quanto maior for o escore, o fumante apresenta menor sensação de abstinência. Esse fato foi relevante na preparação dos dados para a análise estatística, quando se inverteu o escore das questões descritas antes de se proceder com a mesma.
A tradução da versão original da WSWS para o português falado no Brasil foi realizada por um tradutor brasileiro com alto nível de fluência e conhecimento da língua inglesa. Essa tradução para o português foi submetida à tradução retrógrada para o inglês por um tradutor nativo da língua inglesa com alta fluência e conhecimento da língua portuguesa. Ambos desconheciam a escala original.
Para analisar a adaptação cultural, foi constituído um comitê de juízes formado por um especialista com domínio do tema e da área de pesquisa da escala, bem como dos dois idiomas; pelos autores da presente pesquisa; e pelo autor da escala na versão original para analisar possíveis adaptações, sem mudar a essência da escala.
A escala traduzida e adaptada foi aplicada em 8 voluntários fumantes com a finalidade de verificar se as questões eram de fácil compreensão e levantar dúvidas em relação ao texto. A versão final da escala em português foi concluída após a adaptação cultural e sua submissão ao autor original.
Para a avaliação da reprodutibilidade, foi determinada uma amostra por conveniência de 75 voluntários fumantes, baseada no estudo de Hopkins,(12) selecionados e avaliados no Ambulatório do Núcleo de Apoio à Prevenção e Cessação do Tabagismo (PREVFUMO) da UNCISAL. O número de participantes nessa de amostra é adequado de acordo com estudos prévios de adaptação cultural e reprodutibilidade de questionários relacionados a doenças respiratórias no Brasil, como o Saint George's Respiratory Questionnaire(13) e o Questionário de Vias Aéreas 20,(14) nos quais foram avaliados apenas 30 pacientes.
Os critérios de inclusão foram os seguintes: voluntários de ambos os gêneros, maiores de 18 anos, que decidiram abandonar a dependência ao tabaco e com bom nível cognitivo, avaliado pelo Miniexame do Estado Mental (MEM).(15) Os critérios de exclusão foram os seguintes: indivíduos com história de infarto agudo do miocárdio ou acidente vascular cerebral nos três meses precedentes; indivíduos com doenças crônicas não controladas, malignas ou incapacitantes; gestantes; indivíduos com escore no MEM menor que 21; e indivíduos com dosagem de monóxido de carbono no ar expirado (COex) 6 ppm.(8)
Inicialmente, foi avaliada a compreensão dos participantes através da aplicação do questionário MEM. Em seguida, preencheu-se o formulário de coleta de dados sobre dados demográficos, além do questionário Critério de Classificação Econômica Brasil.
No primeiro dia da avaliação do fumante no PREVFUMO (T0), a tradução final da WSWS foi respondida, concomitantemente ao teste de Fagerström,(16) escala hospital anxiety and depression (HAD)(17) e mensuração dos níveis de COex.(18,19) No mesmo dia, a WSWS foi aplicada pela segunda vez (T1), 30 min após T0, por um pesquisador diferente, com o objetivo de avaliar a reprodutibilidade interobservador. Quinze dias após T0, quando os voluntários ainda não haviam iniciado o tratamento para a cessação do tabagismo, a escala foi aplicada pela terceira vez (T2) pelo mesmo pesquisador da primeira avaliação para avaliar a reprodutibilidade intraobservador. O início da terapia para cessação não foi informado aos voluntários, visando minimizar sintomas de ansiedade e/ou depressão.
Sempre antes da cada avaliação, foi realizada a dosagem de COex por meio do aparelho SmokeCheck® (MicroDirect Inc. Lewiston, ME, EUA) a fim de determinar se do voluntário era realmente tabagista, usando-se como ponto de corte valores > 6 ppm,(8) e classificar que tipo de fumante esse era, de acordo com os valores obtidos: fumante leve (7-10 ppm), moderado (11-20 ppm) ou forte (> 20 ppm).(17) Para a determinação do COex, foi solicitado ao voluntário que fizesse uma pausa inspiratória de vinte segundos, permitindo um maior equilíbrio entre o monóxido de carbono no sangue e o no ar alveolar, melhorando a acurácia da medida. Após essa pausa, o voluntário foi orientado a exalar lenta e completamente, com os lábios cerrados em torno do bocal do aparelho para evitar o escape de ar.(19)
Na análise estatística, as variáveis categóricas foram resumidas em frequências absolutas e relativas (porcentagens). As informações referentes a variáveis numéricas foram expressas em médias, desvios-padrão, medianas, valores mínimos e valores máximos. Utilizou-se o teste do qui-quadrado para verificar a relação de dependência entre duas variáveis de distribuição normal. O teste t de Student foi utilizado para a comparação entre duas variáveis independentes de distribuição normal. O teste de Mann-Whitney foi utilizado para comparar duas variáveis independentes de distribuição não normal.(20)
Utilizou-se o coeficiente de correlação intraclasse (CCI) para medir a reprodutibilidade da WSWS. O CCI varia de 0 a 1, sendo que quanto mais próximo de 1, maior a reprodutibilidade da variável. Para medir o grau de concordância entre duas avaliações ordenadas em categorias (questões da escala), utilizou-se o coeficiente de confiabilidade kappa. Por fim, o coeficiente de correlação de Spearman foi utilizado para medir a correlação entre duas variáveis minimamente ordinais. Esse teste não paramétrico foi utilizado levando-se em consideração a natureza das distribuições das variáveis estudadas ou a variabilidade das medidas efetuadas.(21)
Para todos os testes estatísticos, o nível de significância adotado foi erro tipo I < 0,05 ou 5%. A análise foi realizada através do programa Statistical Package for the Social Sciences, versão 13.0 (SPSS Inc., Chicago, IL, EUA).
ResultadosApós a primeira tradução da escala, foram avaliados 8 voluntários, sendo 6 do gênero masculino. A média de idade foi de 38,0 ± 11,2 anos. A WSWS foi bem compreendida, não havendo questionamentos. Apenas o tempo verbal das questões foi alterado de "sinto-me" para "eu tenho sentido". Essa alteração foi aplicada em outros 30 voluntários, sendo escolhida a forma "eu tenho sentido". O tempo final de resposta da escala não foi alterado e nem sua compreensão. A modificação do tempo verbal das questões se deu por motivo de melhor adaptação ao português falado no Brasil. O tempo de resposta foi cronometrado por dois pesquisadores, com tempo total de 6 min e 44 s. A resposta a cada questão variou de 4,2 a 12,6 s (dp = 4,18 s).
Para a reprodutibilidade, foram avaliados 76 voluntários fumantes que procuraram o PREVFUMO. Apenas 1 voluntário não foi incluído na pesquisa por apresentar escore menor que 21 pontos ao MEM. Dos 75 voluntários incluídos, a maioria era do gênero feminino, possuía pelo menos o ensino médio completo e pertenciam à classe econômica A ou B (Tabela 1).

A média de idade dos voluntários foi de 46,3 ± 10,5 anos. O índice de massa corpórea caracterizou a população estudada como tendo sobrepeso mas muito próximo dos valores de normalidade. A média da carga tabágica dos participantes foi de 28,5 ± 18,5 anos-maço, com baixa dependência à nicotina (Tabela 2).
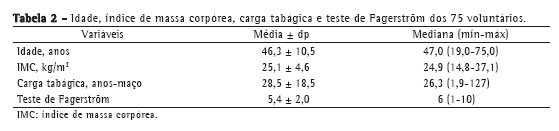
Não houve diferenças estatisticamente significativas entre os gêneros em relação a escolaridade (p = 0,17) ou classificação econômica (p = 0,79), avaliadas pelo teste do qui-quadrado. Em relação à dosagem de COex, 65,3% dos voluntários foram classificados como fumantes fortes (> 20 ppm).
Não houve diferenças significativas entre os gêneros em relação ao grau de dependência nicotínica (p = 0,60) e à carga tabágica (p = 0,57), avaliadas pelo teste de Mann-Whitney. Da mesma forma, os resultados da escala HAD-ansiedade e HAD-depressão não se correlacionaram com o teste de Fagerström, avaliados pelo coeficiente de correlação de Spearman.
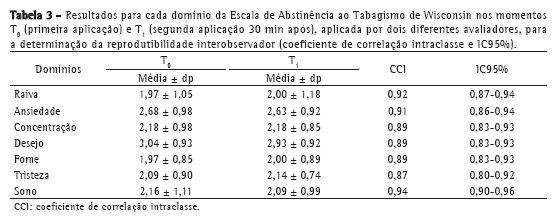
A Tabela 3 demonstra os valores de média e desvio-padrão, bem como os valores mínimos e máximos, para cada domínio da WSWS, nos momentos T0 e T1, sendo a escala aplicada por pesquisadores diferentes (reprodutibilidade interobservador). Observou-se que os valores de CCI foram maiores que 0,75 e que o IC95% variou entre 0,80 e 0,93, demonstrando excelente correlação intraclasse.
A Tabela 4 demonstra os valores médios, o CCI e o IC95% para cada domínio da WSWS, nos momentos T0 e T2, sendo a escala aplicada pelo mesmo avaliador (reprodutibilidade intraobservador). Observou-se que todos os valores de CCI foram maiores que 0,75; portanto, a reprodutibilidade intraobservador obteve concordância excelente, embora os limites inferiores do IC95% foram menores que 0,75 para os domínios raiva, ansiedade, concentração e tristeza.

O coeficiente de confiabilidade kappa foi utilizado para avaliar a reprodutibilidade questão a questão da WSWS. A maioria das questões apresentou nível de concordância de moderado a bom, sendo o coeficiente menor que 0,4 apenas nas questões 18 e 27 (Tabela 5).
 Discussão
DiscussãoA versão brasileira da WSWS apresentou excelente reprodutibilidade, com valores de CCI maiores que 0,76 em todos os domínios, demonstrando excelente concordância entre as respostas. Os escores obtidos, tanto na aplicação interobservador, quanto na intraobservador, foram semelhantes, confirmando que os diferentes observadores não influenciaram as respostas dos voluntários. Além disso, a análise da concordância kappa apresentou ótimos resultados, com valores acima de 0,4 em 92,9% das questões da escala.(21)
A WSWS é autoaplicável, e os voluntários não apresentaram dificuldade para entender ou responder as questões. Os mesmos apresentaram MEM acima de 21 pontos e pertenciam às classes sociais A ou B em sua maioria, fato que pode ter influenciado diretamente nessa compreensão. A análise da reprodutibilidade foi feita antes da terapia para a cessação do tabagismo para evitar que fatores relacionados à abstinência influenciassem a reprodutibilidade.(22) Em caso de voluntários com dificuldade em leitura, o questionário foi lido e parcialmente explicado pelo avaliador.
A carga tabágica não se correlacionou com os resultados do teste de Fagerström. A literatura apresenta resultados semelhantes em alguns estudos, que demonstraram uma fraca associação entre essas variáveis, indicando que a quantidade de cigarros consumida durante um ano não se associa diretamente ao grau de dependência à nicotina.(21) Outros estudos resultaram em ótimas associações entre essas variáveis, mostrando que esses achados são controversos porém relevantes para projetos de estudos posteriores.(5,16) Deve ser levado em conta que a carga tabágica relatada pelo voluntário pode não ser confiável, pois muitos fumantes, por viés de memória ou mesmo deliberadamente, podem subestimar o número de cigarros consumidos.(23)
Obteve-se fraca correlação entre o COex e o escore do teste de Fagerström. Observa-se que os voluntários da presente pesquisa, em média, apresentaram baixa dependência à nicotina mas, em sua maioria, apresentavam níveis de COex que os classificou como fumantes fortes. Esses resultados estão de acordo com diversos estudos nos quais os voluntários apresentaram baixa dependência à nicotina (mediana de 5) e elevados níveis de COex (> 20 ppm), indicando uma fraca correlação entre essas variáveis e ressaltando a complexidade de se quantificar a dependência à nicotina.(23-25) Especificamente no presente estudo, acredita-se que a ansiedade gerada pelo iminente ingresso em um grupo de cessação do tabagismo tenha levado os voluntários a fumar mais que o normal nas horas precedentes à entrevista, o que elevou os níveis de COex.
A literatura confirma um aumento dos afetos negativos em fumantes,(26,27) bem como elevados níveis de ansiedade e depressão naqueles que estão prestes a entrar num programa de cessação do tabagismo.(28,29) Julga-se que o fato de o nosso centro ser referência na cessação do tabagismo possa ter influenciado na procura dos voluntários que sabidamente possuíam níveis elevados de ansiedade e depressão, principalmente, pelos insucessos na tentativa de parar de fumar.
Não se observou uma correlação significativa dos escores da escala HAD com o grau de dependência nicotínica. Esses achados estão de acordo com estudos que relataram a ausência de correlação entre a tendência à depressão (HAD) e dependência à nicotina (Fagerström).(28,29) Entretanto, há divergências quanto a esse aspecto em estudos que relataram correlação positiva e significativa entre ansiedade, depressão e dependência nicotínica, concordando com o DSM-IV.(5,30) A discordância entre os estudos talvez resida nos instrumentos de avaliação utilizados, os quais são bastante distintos, concordando com um estudo que encontrou pobre concordância entre o teste de Fagerström e o DSM-IV. Os autores comentam que o DSM-IV consegue classificar a dependência à nicotina sob aspectos dos sintomas psiquiátricos, enquanto que o teste de Fagerström levaria mais em conta o prazer de fumar. Assim, a estimativa de dependência nicotínica deve ser multifatorial, sendo incapaz, portanto, de ser realizada por um único instrumento.(30)
Em conclusão, a presente pesquisa demonstrou a viabilidade da tradução e adaptação cultural, com excelente reprodutibilidade de um questionário de origem em língua estrangeira. Obteve-se excelente reprodutibilidade intra e interobservador em todos os domínios da Escala de Abstinência ao Tabagismo de Wisconsin. Assim, a versão para o português falado no Brasil da WSWS é reprodutível e de fácil aplicação, podendo ser utilizada para a avaliação dos sintomas da síndrome de abstinência à nicotina, no período pós-cessação do tabagismo, na população brasileira.
Referências1. Guriérrez AJ. El tabaquismo como problema de salud pública. In: Ferrero MB, Mezquita MA, García MT, editors. Manual de prevención y tratamiento del tabaquismo. 3rd ed. Madrid: Ergon; 2003. p. 27-68.
2. Campo-Arias A. The prevalence of nicotine-dependency in some populations: a systematic review [Article in Spanish]. Rev Salud Publica (Bogota). 2006;8(1):98-107. http://dx.doi.org/10.1590/S0124-00642006000100009
3. Hutton JJ, Hackney C. Metabolism of cigarette smoke condensates by human and rat homogenates to form mutagens detectable by Salmonella typhimurium TA1538. Cancer Res. 1975;35(9):2461-8. PMid:1097108.
4. Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25(26):6208-12. PMid:15987950. http://dx.doi.org/10.1523/JNEUROSCI.4785-04.2005
5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994.
6. Picciolo M, Gigante D, Nunziata A. Nicotine addiction and current therapy of smoking cessation [Article in Italian]. Clin Ter. 2005;156(4):159-71. PMid:16342517.
7. Miranda M, Slachevsky A, Venegas P. Delirium from nicotine withdrawal in a post-operative adult patient [Article in Spanish]. Rev Med Chil. 2005;133(3):385-6. PMid:15880196.
8. Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers' gender. Exp Clin Psychopharmacol. 2006;14(2):121 35. PMid:16756416 PMCid:1564049. http://dx.doi.org/10.1037/1064-1297.14.2.121
9. Otero UB, Perez Cde A, Szklo M, Esteves GA, dePinho MM, Szklo AS, et al. Randomized clinical trial: effectiveness of the cognitive-behavioral approach and the use of nicotine replacement transdermal patches for smoking cessation among adults in Rio de Janeiro, Brazil [Article in Portuguese]. Cad Saude Publica. 2006;22(2):439 49. PMid:16501756. http://dx.doi.org/10.1590/S0102-311X2006000200021
10. Etter JF, Le Houezec J, Perneger TV. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology. 2003;28(2):359-70. PMid:12589389. http://dx.doi.org/10.1038/sj.npp.1300030
11. Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB.
Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354-61. PMid:10609970. http://dx.doi.org/10.1037/1064-1297.7.4.354
12. Hopkins WG. Measures of reliability in sports medicine and science. Sports Med. 2000;30(1):1-15. PMid:10907753. http://dx.doi.org/10.2165/00007256-200030010-00001
13. Souza TC, Jardim JR, Jones P. Validação do Questionário do Hospital Saint George na Doença Respiratória (SGRQ) em pacientes portadores de doença pulmonar obstrutiva crônica no Brasil. J Pneumol. 2000;26(3):119-28. http://dx.doi.org/10.1590/S0102-35862000000300004
14. Camelier A, Rosa FW, Jones PW, Jardim JR. Brazilian version of airways questionnaire 20: a reproducibility study and correlations in patients with COPD. Respir Med. 2005;99(5):602-8. PMid:15823458. http://dx.doi.org/10.1016/j.rmed.2004.09.022
15. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189 98. http://dx.doi.org/10.1016/0022-
3956(75)90026-6
16. Carmo JT, Pueyo AA. A adaptação do português do Fagerström Test for Nicotine Dependence (FTND) para avaliar a dependência e tolerância à nicotina em fumantes brasileiros. RBM Rev Bras Med. 2002;59(1/2):73-80.
17. Botega NJ, Bio MR, Zomignani MA, Garcia C Jr, Pereira WA. Mood disorders among inpatients in ambulatory and validation of the anxiety and depression scale HAD [Article in Portuguese].Rev Saude Publica. 1995;29(5):355-63. PMid:8731275.
18. Santos UP, Gannam S, Abe JM, Esteves PB, Filho MF, Wakassa TB, et al. Emprego da determinação de monóxido de carbono no ar exalado para a detecção do consumo de tabaco. J Pneumol. 2001;27(5):231-236. http://dx.doi.org/10.1590/S0102-35862001000500001
19. Reichert J, Araújo AJ, Gonçalves CM, Godoy I, Chatkin JM, Sales MP, et al. Smoking cessation guidelines--2008. J Bras Pneumol. 2008;34(10):845-80. Erratum in: J Bras Pneumol. 2008;34(12):1090. PMid:19009219. http://dx.doi.org/10.1590/S1806-37132008001000014
20. Siegel S. Estatística não-paramétrica para ciências do
comportamento. São Paulo: McGraw Hill; 1981.
21. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-8. http://dx.doi.org/10.1037/0033-2909.86.2.420
22. West R, Ussher M, Evans M, Rashid M. Assessing DSM-IV nicotine withdrawal symptoms: a comparison and evaluation of five different scales. Psychopharmacology (Berl). 2006;184(3-4):619-27. PMid:16308727. http://dx.doi.org/10.1007/s00213-005-0216-z
23. Burling AS, Burling TA. A comparison of self-report measures of nicotine dependence among male drug/alcohol-dependent cigarette smokers. Nicotine Tob Res. 2003;5(5):625-33. http://dx.doi.org/10.1080/1462220031000158708
24. Meneses-Gaya IC, Zuardi AW, Loureiro SR, Crippa JA. Psychometric properties of the Fagerström Test for Nicotine Dependence. J Bras Pneumol. 2009;35(1):73 82. PMid:19219334. http://dx.doi.org/10.1590/S1806-37132009000100011
25. Brown C, Madden PA, Palenchar DR, Cooper-Patrick L. The association between depressive symptoms and cigarette smoking in an urban primary care sample. Int J Psychiatry Med. 2000;30(1):15-26. PMid:10900558. http://dx.doi.org/10.2190/NY79-CJ0H-VBAY-5M1U
26. Karp I, O'Loughlin J, Hanley J, Tyndale RF, Paradis G. Risk factors for tobacco dependence in adolescent smokers. Tob Control. 2006;15(3):199-204. PMid:16728750 PMCid:2564659. http://dx.doi.org/10.1136/tc.2005.014118
27. Cosci F, Schruers KR, Pistelli F, Griez EJ. Negative affectivity in smokers applying to smoking cessation clinics: a case-control study. Depress Anxiety. 2009;26(9):824-30. PMid:19105219. http://dx.doi.org/10.1002/da.20473
28. Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. Am J Public Health. 2000;90(7):1122-7. http://dx.doi.org/10.2105/AJPH.90.7.1122
29. Psujek JK, Martz DM, Curtin L, Michael KD, Aeschleman SR. Gender differences in the association among nicotine dependence, body image, depression, and anxiety within a college population. Addict Behav. 2004;29(2):375 80. PMid:14732426. http://dx.doi.org/10.1016/j.addbeh.2003.08.031
30. Spada MM, Nikcević AV, Moneta GB, Wells A. Metacognition as a mediator of the relationship between emotion and smoking dependence. Addict Behav. 2007;32(10):2120-9. PMid:17307299. http://dx.doi.org/10.1016/j.addbeh.2007.01.012
* Trabalho realizado na Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
Endereço para correspondência: Ilka L. Santoro. Disciplina de Pneumologia, Rua Botucatu, 740, 3º andar, CEP 04023-062, São Paulo, SP, Brasil.
Tel. 55 11 5576-4238. Email: ilka@pneumo.epm.br
Apoio financeiro: Nenhum.
Recebido para publicação em 11/4/2012. Aprovado, após revisão, em 20/9/2012.
Sobre os autoresBoanerges Lopes de Oliveira Junior
Fisioterapeuta Assistencial. Universidade Estadual de Ciências da Saúde de Alagoas - UNCISAL - Maceió (AL) Brasil.
José Roberto Jardim
Professor Adjunto Livre-Docente. Disciplina de Pneumologia, Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
Oliver Augusto Nascimento
Médico Assistente. Disciplina de Pneumologia, Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
George Márcio da Costa e Souza
Professor Assistente. Curso de Fisioterapia, Universidade Estadual de Ciências da Saúde de Alagoas - UNCISAL - Maceió (AL) Brasil.
Timothy B. Baker
Director of Research. Center for Tobacco Research and Intervention, University of Wisconsin, Madison (WI) EUA.
Ilka Lopes Santoro
Professora Afiliada. Disciplina de Pneumologia, Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.






