ABSTRACT
Objective: Levels of procalcitonin, midregional pro-atrial natriuretic peptide (MR-proANP), C-terminal provasopressin (copeptin), and C-reactive protein (CRP), as well as Sequential Organ Failure Assessment (SOFA) scores, are associated with severity and described as predictors of outcome in ventilator-associated pneumonia (VAP). This study sought to compare the predictive value of these biomarkers for mortality in VAP. Methods: An observational study of 71 patients with VAP. Levels of procalcitonin, MR-proANP, copeptin, and CRP, together with SOFA scores, were determined at VAP onset, designated day 0 (D0), and on day 4 of treatment (D4). Patients received empirical antimicrobial therapy, with modifications based on culture results. Patients who died before D28 were classified as nonsurvivors. Results: Of the 71 patients evaluated, 45 were classified as survivors. Of the 45 survivors, 35 (77.8%) received appropriate antimicrobial therapy, compared with 18 (69.2%) of the 26 nonsurvivors (p = 0.57). On D0 and D4, the levels of all biomarkers (except CRP), as well as SOFA scores, were lower in eventual survivors than in eventual nonsurvivors. For D0 and D4, the area under the ROC curve was largest for procalcitonin. On D0, MR-proANP had the highest positive likelihood ratio (2.71) and positive predictive value (0.60), but procalcitonin had the highest negative predictive value (0.87). On D4, procalcitonin had the highest positive likelihood ratio (3.46), the highest positive predictive value (0.66), and the highest negative predictive value (0.93). Conclusions: The biomarkers procalcitonin, MR-proANP, and copeptin can predict mortality in VAP, as can the SOFA score. Procalcitonin alone has the greatest predictive power for such mortality.
Keywords:
Pneumonia, ventilator-associated/mortality; Biological markers/analysis; Health Status Indicators.
RESUMO
Objetivo: Níveis de procalcitonina, midregional pro-atrial natriuretic peptide (MR-proANP, pró-peptídeo natriurético atrial midregional),, C-terminal provasopressin (copeptina), proteína C reativa (CRP) e escore do Sequential Organ Failure Assessment (SOFA) são associados a gravidade e descritos como preditores de desfechos na pneumonia associada a ventilação mecânica (PAVM). Este estudo procurou comparar o valor preditivo de mortalidade desses biomarcadores na PAVM. Métodos: Estudo observacional com 71 pacientes com PAVM. Níveis de procalcitonina, MR-proANP, copeptina e PCR, bem como escore de SOFA foram obtidos no dia do diagnóstico de PAVM, designado dia zero (D0), e no quarto dia de tratamento (D4) Os pacientes receberam tratamento antimicrobiano empírico, com modificações baseadas nos resultados de cultura. Os pacientes que morreram antes de D28 foram classificados como não sobreviventes. Resultados: Dos 71 pacientes, 45 sobreviveram. Dos 45 sobreviventes, 35 (77,8%) receberam tratamento antimicrobiano adequado, comparados com 18 (69,2%) dos 26 não sobreviventes (p = 0,57). Os sobreviventes apresentaram valores significativamente mais baixos em todos os biomarcadores estudados, inclusive no escore de SOFA (exceto PCR) em D0 e D4. Em D0 e D4, a área sob a curva ROC foi maior para procalcitonina. Em D0, MR-proANP teve a maior razão de verossimilhança positiva (2,71) e valor preditivo positivo (0,60), mas a procalcitonina apresentou o maior valor preditivo negativo (0,87). Em D4, a procalcitonina apresentou a maior razão de verossimilhança positiva (3,46), o maior valor preditivo positivo (0,66) e o maior valor preditivo negativo (0,93). Conclusões: Os biomarcadores procalcitonina, MR-proANP e copeptina podem predizer mortalidade na PAVM, assim como o escore de SOFA. A procalcitonina tem o maior poder preditivo de mortalidade na PAVM.
Palavras-chave:
Pneumonia associada à ventilação mecânica/mortalidade; Marcadores Biológicos/análise; Indicadores básicos de saúde.
IntroductionDespite recent technological advances, the mortality related to ventilator-associated pneumonia (VAP) remains high. The mortality rate in patients with VAP generally ranges from 24% to 50% and can reach 76% in specific settings or when lung infection is caused by high-risk pathogens.(1) Previous studies have demonstrated that this mortality rate is high in Brazil.(2,3) High mortality rates in patients with VAP have been attributed to multiple organ dysfunction syndrome, infection with certain multidrug-resistant bacteria, and inappropriate antibiotic therapy.(4) The scoring system known as the Sequential Organ Failure Assessment (SOFA), originally named "Sepsis-Related Organ Failure Assessment", was developed to describe morbidity, rather than to predict mortality, and its use was primarily limited to critically ill ICU patients with sepsis. However, the score was later re-evaluated, coming to be used in other populations of critically ill patients and as a predictor of mortality.(5)
Various substances, including procalcitonin, midregional pro-atrial natriuretic peptide (MR-proANP), C-terminal provasopressin (copeptin), and C-reactive protein (CRP), have been studied as biomarkers of prognosis and treatment response in patients with VAP.
In an earlier, prospective observational cohort study, the prognostic value of procalcitonin kinetics was investigated in patients with VAP.(6) The authors found that elevated serum levels of procalcitonin on days 1, 3, and 7 of VAP were strong predictors of unfavorable outcome. Another prospective observational study demonstrated that decreasing values of either procalcitonin or CRP, from VAP onset to day 4 of treatment, were independent predictors of survival in patients with VAP.(7) Copeptin levels have been shown to increase in parallel with increases in the severity of sepsis and to be independent predictors of mortality in VAP, patients with VAP accompanied by septic shock presenting the highest copeptin levels and the highest mortality.(8) The levels of MR-proANP also increase as sepsis severity increases and are independent predictors of mortality in VAP.(9)
The aim of this study was to compare the predictive value of the levels of the biomarkers procalcitonin, CRP, copeptin, and MR-proANP, as well as that of the SOFA score, for mortality in patients with VAP. Although some of the data presented in this study have been published in previous studies,(7-9) those studies did not compare the predictors of mortality in terms of their accuracy.
MethodsThis was an observational cohort study conducted in the 26-bed ICU of a 744-bed tertiary-care teaching hospital, involving consecutive patients suspected of having VAP upon admission to the ICU of the Hospital de Clínicas de Porto Alegre (HCPA, Porto Alegre Hospital de Clínicas), in the city of Porto Alegre, Brazil. The exclusion criteria were being < 18 years of age; having previously been diagnosed with AIDS; and presenting with neutropenia (< 1,000 cells/mL). The study sample consisted of 71 patients admitted to the ICU and developing VAP between October of 2003 and August of 2005. The day of VAP onset was designated day 0 (D0). Biomarkers were determined in samples that had been collected on D0 or D4 and had been frozen for future biochemical analysis.
Pneumonia was classified as VAP when it occurred after 48 h on mechanical ventilation and was therefore judged not to have been incubating before the initiation of the mechanical ventilation. Early-onset VAP was defined as that occurring during the first 4 days on mechanical ventilation, whereas VAP developing thereafter was classified as late-onset.(10) Acute Physiology and Chronic Health Evaluation II (APACHE II) scores(11) were calculated within the first 24 h after ICU admission. Patients having received chemotherapy within the last 45 days were categorized as being immunosuppressed. A diagnosis of pneumonia was suspected when new, persistent infiltrate was seen on chest X-rays and at least two of the following were observed: a body temperature below 36°C or above 38°C; a white blood cell count lower than 4,000/mm3 or higher than 11,000/mm3; and macroscopically purulent tracheal aspirate. Tracheal aspirate was classified as purulent or nonpurulent after visual inspection by the clinical treatment team. The highest axillary temperature recorded during the 24 h preceding inclusion in the study was registered. Chest X-rays, arterial blood gas analyses, and complete blood counts, as well as the values of creatinine, total bilirubin, and albumin, were obtained for D0 and D4. Tracheal aspirate for quantitative culture had been obtained on D0, before antimicrobial treatment was started. For D0, we calculated the clinical pulmonary infection score (CPIS), modified as described by Singh et al.,(12) adding points for microbiological results and progression of pulmonary infiltrate seen on an additional chest X-ray taken on D3. Patients were assumed to have VAP when the CPIS was 7 or more. Patients received a diagnosis of VAP only after other medical conditions to which the presenting symptoms, signs or radiological findings could be attributed had been ruled out. Tracheal aspirate cultures yielding ≥ 105 CFU/mL were considered positive. For all patients in whom the clinical suspicion of VAP was confirmed, empirical antimicrobial therapy was started on D0. The antibiotic therapy was selected by the critical care team or primary care team, as were any decisions regarding changes in that therapy. The SOFA score was calculated for D0 and D4. The pre-sedation Glasgow Coma Score was used to evaluate the neurologic status in patients under sedation.(13) Modifications to the empirical therapy were based on the results of tracheal aspirate cultures and blood cultures. Airway management was performed in accordance with a standard protocol in all patients. At the time of VAP diagnosis, patients were classified as having sepsis, severe sepsis, or septic shock, as defined in international guidelines.(14) Patients were followed until D28, at which point crude mortality was assessed. Patients discharged from the ICU before D28 were classified as survivors. The records of all patients with VAP were reviewed by one of the investigators in order to confirm the diagnosis on the basis of predetermined criteria. The research protocol was reviewed and approved by the Human Research Ethics Committee of the HCPA. Written informed consent was obtained, prior to enrollment, from the representatives of the patients. The study protocol conforms to the ethical guidelines established in the Declaration of Helsinki. On D0, D3, D4, and weekly until D28, trained investigators collected data related to the following: age; gender; APACHE II score; SOFA score; CPIS; presence of COPD; smoking status; history of congestive heart failure; history of malignancy; immunosuppression; albumin level; use of H2 antagonists; proton pump inhibitor use; corticosteroid use; and the need for dialysis. Data related to the following factors were registered but are not shown here: cause of ICU admission; PaO2/FiO2 ratio; central venous catheterization; urinary tract catheterization; duration of mechanical ventilation; duration of stay in the ICU before VAP; cardiopulmonary resuscitation; intubation (orotracheal or nasotracheal); and tracheotomy. The appropriateness of the empirical antimicrobial treatment was determined on the basis of microbiological results. Appropriate antibiotic therapy was defined as coverage of all of the pathogens isolated (from tracheal aspirate cultures or blood cultures) by at least one antimicrobial administered at the onset of VAP, as determined by the sensitivity pattern observed in the antibiogram. Treatment was considered appropriate when cultures were negative. When a diagnosis of VAP was clinically suspected, blood was drawn before empirical antibiotic therapy was started. Immediately after the blood had been drawn, serum samples were prepared and stored at −80°C in the HCPA research laboratory. Assays were performed in batches at the end of the study period.
Procalcitonin levels were determined with the commercially available immunoluminometric assay (BRAHMS PCT LIA; B R A H M S, Berlin, Germany) with a functional assay sensitivity-defined as lowest value with an interassay coefficient of variance (CV) < 20%-of 0.1 ng/mL, as analyzed with a luminometer (Lumat LB 9507; Berthold, Bad Wildbad, Germany).(15,16) The determination of procalcitonin levels was performed at the HCPA Experimental Research Center. Levels of CRP were measured with a nephelometer (BN II; Dade Behring, Marburg, Germany), as routinely performed in the HCPA Clinical Pathology Laboratory.
Copeptin levels were determined using an immunoluminometric sandwich assay, as previously described,(17) with a functional assay sensitivity of 2.25 pmol/L. Levels of MR-proANP were also measured using an immunoluminometric sandwich assay (BRAHMS MR-proANP LIA; B R A H M S), the functional assay sensitivity of which is 11 pmol/L.(18) Measurements of MR-proANP and copeptin levels were performed in a blinded fashion in the B R A H M S research laboratory. All other laboratory measurements were performed at the HCPA research laboratory by technicians who were blinded to the clinical status and outcomes of the cases.
The statistical analysis was performed with the Statistical Package for the Social Sciences, version 11.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as mean ± SD. Categorical variables were compared by the chi-square test. Differences between survivors and nonsurvivors were analyzed with the Mann-Whitney test. Differences related to septic status were analyzed with the Kruskal-Wallis test. We used ROC curves and the corresponding area under the curve values to assess biomarkers and SOFA score as prognostic markers at D0 and D4. The optimal cut-off value for equal sensitivity and specificity was calculated by the Youden index. All tests were two-tailed, and values of p < 0.05 were considered statistically significant.
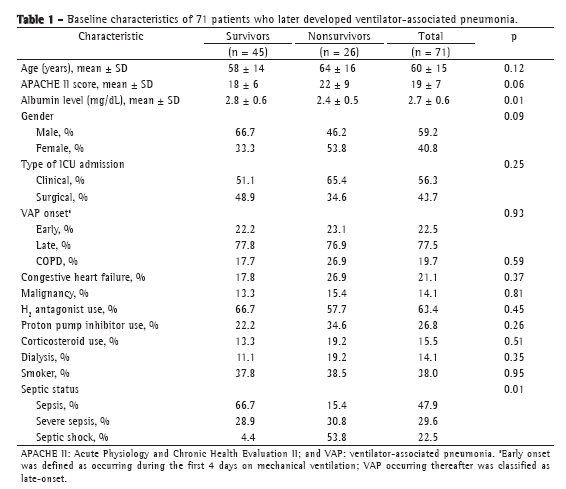
ResultsThe study sample comprised 71 patients with VAP, of whom 45 (63.4%) survived until D28 and 26 (36.6%) did not. The baseline characteristics of the study population are detailed in Table 1. We excluded 8 patients from the D4 analysis, for the following reasons: death before D4 (n = 6); discharge from the ICU before D4 (n = 1); and lack of biomarker data due to the unavailability of a serum sample (n = 1). None of the patients met the criteria for immunosuppression. Fifty-eight patients (81.7%) had received antibiotics in the last 10 days before VAP onset.
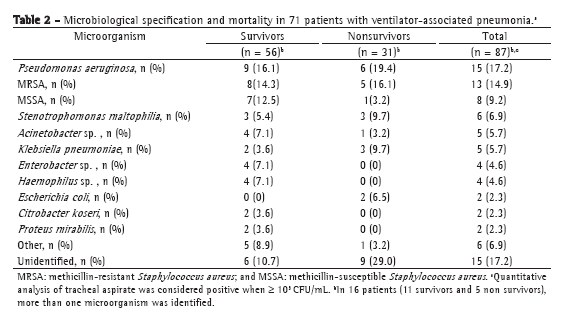
Microbiological specification by tracheal aspirate culture revealed the most common microorganisms, in survivors and nonsurvivors, to be Pseudomonas aeruginosa, Staphylococcus aureus (methicillin-resistant and methicillin-susceptible), Stenotrophomonas maltophilia, Acinetobacter sp., Klebsiella pneumoniae, and Enterobacter sp. In 16 patients (11 survivors and 5 nonsurvivors), more than one microorganism was identified (Table 2).
Of the 71 patients evaluated, 16 (22.5%) developed early-onset VAP and 55 (77.5%) developed late-onset VAP. Among the 16 patients with early-onset VAP, there were 6 deaths (mortality, 37.5%), compared with 20 deaths (mortality, 36.4%) among the 55 patients with late-onset VAP (p = 1.0). Of the 45 survivors, 35 (77.8%) received appropriate antimicrobial therapy, compared with 18 (69.2%) of the 26 nonsurvivors (p = 0.57).
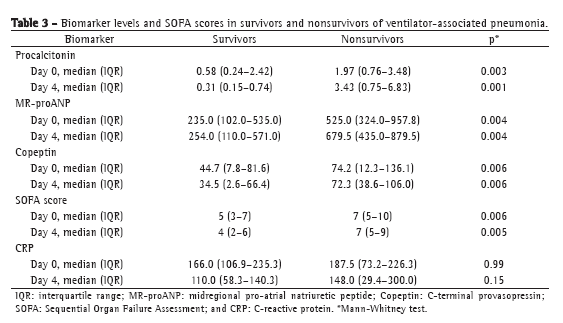
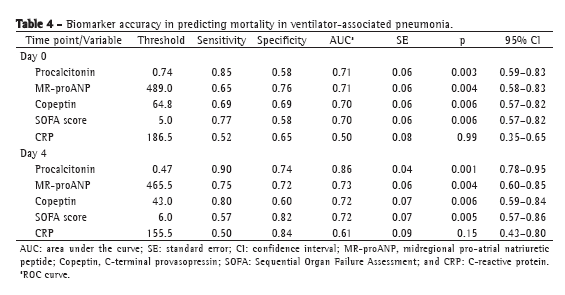
The levels of most of the biomarkers studied, as well as the SOFA scores, were lower in the patients with sepsis than in those with severe sepsis or septic shock: procalcitonin (p = 0.0001 for D0 and p = 0.001 for D4); MR-proANP (p = 0.002 for D0 and p = 0.02 for D4); copeptin (p = 0.001 for D0 and p = 0.009 for D4); and SOFA score (p = 0.0001 for D0 and p = 0.01 for D4). The same was true for the comparison between survivors and nonsurvivors, the values being lower in the former. In both cases, the exception was CRP. Although CRP values were lower in survivors than in nonsurvivors, it did not significantly discriminate between the two groups (Table 3). The ROC curve data for mortality on D0 and on D4 are shown in Table 4. Procalcitonin levels showed the largest area under the curve for D0 and D4. For D4, the sensitivity and specificity of procalcitonin for predicting mortality were 90% and 74%, respectively. Positive predictive values (PPVs), negative predictive values (NPVs), and likelihood ratios for mortality are shown in Table 5. In D0 samples, MR-proANP had the highest PPV for mortality (0.60), whereas procalcitonin had the highest NPV (0.87). In D4 samples, procalcitonin had the highest PPV (0.66), as well as the highest NPV (0.93), for mortality.
 Discussion
DiscussionThe expression of serum markers can vary when individuals are exposed to bacterial toxins, and the stimulation can be multifactorial. Changes in the levels of biomarkers can signal a change in clinical status.
Considering the mortality rate of VAP, it is highly desirable to have laboratory markers that are early predictors of outcome or of the need to reassess the initial empirical antimicrobial therapy.
In patients with VAP, distinct mechanisms related to inflammation, toxemia, cardiovascular dysfunction, and hypotension result in the production of the biomarkers assessed in the present study. The levels of those biomarkers increase in parallel with increases in the severity of infection. High levels of procalcitonin, MR-proANP, and copeptin, as well as high SOFA scores, have previously been demonstrated to be associated with mortality,(7-9) and those values increase progressively as patient status worsens (from sepsis to severe sepsis to septic shock). In the present study, we found that, for all biomarkers, the NPV was higher than the PPV. This could have important clinical implications, because lower biomarker values (higher NPV) might signal lower mortality risk.
Our cohort was composed of patients who, at VAP onset, had sepsis, severe sepsis, or septic shock. In this group of patients, many of whom presented with multiple organ dysfunction, high levels of procalcitonin, CRP, MR-proANP, and copeptin would be expected, especially in the patients with cardiovascular dysfunction and hypotension.
It is well known that ANP is predominantly produced in the atrium of the heart, regulates a variety of physiological parameters, including diuresis and natriuresis, as well as reducing systemic blood pressure.(19) The levels of ANP seem to be more determined by the intrinsic myocardial depression of sepsis. In addition to sepsis-induced myocardial depression, acute lung injury and an increased afterload placed on the right heart following pulmonary hypertension can contribute to cardiac dilation and a rise in circulating ANP levels.(20) The determination of plasma MR-proANP levels has recently emerged as a valuable tool for individual risk assessment in patients with sepsis.(21) Levels of ANP might reflect the inflammatory cytokine response correlated with the severity of pneumonia, as well as the presence of relevant comorbidities, namely heart failure and renal dysfunction.(22)
Arginine vasopressin (AVP), is released following different stimuli, such as hypotension, hypoxia, hyperosmolarity, acidosis, and infection.(23) It has vasoconstrictor and antidiuretic properties, as well as the capacity to restore vascular tone in vasodilatory hypotension. Copeptin is a AVP precursor and its concentration mirrors that of AVP, both being elevated in sepsis and septic shock.(24) In critically ill patients, there is a strong positive correlation between copeptin values and disease severity. In our patients with sepsis, baseline copeptin values were higher in nonsurvivors than in survivors. This suggests that copeptin represents a prognostic marker in sepsis.(25)
Previous studies, in patients with community-acquired pneumonia (CAP), have demonstrated the predictive value of biomarkers, showing that the levels of MR-proANP, although not those of CRP, gradually increase in parallel with increases in the severity of CAP. In terms of its accuracy as a predictor of survival, the MR-proANP level has been found to be similar to the pneumonia severity index (PSI) and better than procalcitonin level, CRP level, or leukocyte count.(26) In another study, the PSI was found to correlate positively with MR-proANP level and copeptin level.(27) Those authors found that MR-proANP and copeptin levels were significantly higher in nonsurvivors than in survivors. In another study, involving 589 patients with proven CAP, the levels of MR-proANP, copeptin, CRP, and procalcitonin, as well as a mortality prediction score, were determined at admission. The authors found MR-proANP level and copeptin level to be the strongest predictors of mortality. Levels of MR-proANP and copeptin were significantly higher in CAP nonsurvivors and correlated with disease severity as quantified by the mortality prediction score.(28) Although our study involved patients with VAP, our results show some similarities to data presented in these studies of CAP.(27,28) In our study, the levels of procalcitonin, MR-proANP, and copeptin were also significantly higher in the nonsurvivors.
Our findings for D0 indicate the severity of the disease before the initiation of antibiotic therapy. Patients with biomarker values above the threshold for mortality on D0 were at a higher risk for mortality before antimicrobial treatment for VAP. However, our findings for D4 show the intensity of biomarker expression after 4 days of treatment. Patients with biomarker values above the threshold for mortality on D4 should receive special attention and should be reassessed. We found that the levels of procalcitonin, MR-proANP, and copeptin differentiated between survivors and nonsurvivors at both of the time points evaluated, as did the SOFA score. Although CRP has been described as a useful parameter to support the diagnosis of infection(29) and as an indicator of sepsis resolution,(30) absolute CRP values did not differentiate between survivors and nonsurvivors at either of the time points evaluated in our study (p = 0.99 and p = 0.15, respectively).
In our sample, an MR-proANP level above the threshold of 489.0 pmol/L showed the highest accuracy for predicting mortality at VAP onset. The level of MR-proANP had the highest positive likelihood ratio for mortality on D0 and the highest PPV (0.60). As would be expected, patients with severe organ dysfunction were at a higher risk for mortality, and high MR-proANP levels are indicative of major cardiovascular dysfunction. However, for D0, procalcitonin had the highest NPV (0.87), lower levels indicating lower mortality risk at that point.
We observed a change in biomarker accuracy on D4, when procalcitonin showed the highest PPV for mortality. A procalcitonin level above the threshold of 0.47 ng/mL showed the highest accuracy for predicting mortality. On D4, procalcitonin had the highest positive likelihood ratio (3.46), the highest PPV (0.66), and the highest NPV (0.93). The high procalcitonin levels on D4 suggest a continuous stimulus for its expression and the maintenance of the septic focus, which would indicate failure of infection control.
Our data should be interpreted in view of certain limitations. The small size of our sample limited the power of our analysis. A clinical diagnosis of VAP supported by the CPIS has certain limitations, and quantitative culture of tracheal aspirate is a source of ongoing debate in the medical literature, mainly in patients who have previously received antimicrobial therapy.
Although the use of crude mortality rates rather than attributable mortality rates could be interpreted as another limitation, it avoids variability, as well as confounding factors, and is typically used in studies of VAP.
Finally, MR-proANP levels showed the highest accuracy for mortality prediction on D0. We can speculate that this biomarker best detects severity in the first hours of VAP, reflecting a high degree of cardiovascular dysfunction and the consequent mortality risk. However, procalcitonin was the best biomarker for mortality prediction on D0 and D4, showing the highest accuracy on D4. Procalcitonin expression decreases as the infection is brought under control.
Early identification of patients at high risk can provide an opportunity to change the treatment strategy. Additional studies are warranted in order to corroborate our findings and to further define the potential impact of strategies based on biomarkers, perhaps a combined score, to improve VAP outcomes.
AcknowledgmentsWe would like to thank the Grupo de Pesquisa e Pós-Graduação (GPPG, Graduate Research Group) of the HCPA for their support in the execution of this study.
References1. Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867-903.
2. Guimarães MM, Rocco JR. Prevalence of ventilator-associated pneumonia in a university hospital and prognosis for the patients affected. J Bras Pneumol. 2006;32(4):339-46.
3. Rodrigues PM, Carmo Neto E, Santos LR, Knibel MF. Ventilator-associated pneumonia: epidemiology and impact on the clinical evolution of ICU patients. J Bras Pneumol. 2009;35(11):1084-91.
4. Kollef MH, Wragge T, Pasque C. Determinants of mortality and multiorgan dysfunction in cardiac surgery patients requiring prolonged mechanical ventilation. Chest. 1995;107(5):1395-401.
5. Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ
dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793-800.
6. Luyt CE, Guérin V, Combes A, Trouillet JL, Ayed SB, Bernard M, et al. Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2005;171(1):48-53.
7. Seligman R, Meisner M, Lisboa TC, Hertz FT, Filippin TB, Fachel JM, et al. Decreases in procalcitonin and C-reactive protein are strong predictors of survival in ventilator-associated pneumonia. Crit Care. 2006;10(5):R125.
8. Seligman R, Papassotiriou J, Morgenthaler NG, Meisner M, Teixeira PJ. Copeptin, a novel prognostic biomarker in ventilator-associated pneumonia. Crit Care. 2008;12(1):R11.
9. Seligman R, Papassotiriou J, Morgenthaler NG, Meisner M, Teixeira PJ. Prognostic value of midregional pro-atrial natriuretic peptide in ventilator-associated pneumonia. Intensive Care Med. 2008;34(11):2084-
91.
10. Langer M, Cigada M, Mandelli M, Mosconi P, Tognoni G. Early onset pneumonia: a multicenter study in intensive care units. Intensive Care Med. 1987;13(5):342-6.
11. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-29.
12. Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(2 Pt 1):505-11.
13. Peres Bota D, Melot C, Lopes Ferreira F, Nguyen Ba V, Vincent JL. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med. 2002;28(11):1619-24.
14. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644-55.
15. Gendrel D, Bohuon C. Procalcitonin, a marker of bacterial infection. Infection. 1997;25(3):133-4.
16. Karzai W, Oberhoffer M, Meier-Hellmann A, Reinhart K. Procalcitonin--a new indicator of the systemic response to severe infections. Infection. 1997;25(6):329-34.
17. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112-9.
18. Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem. 2004;50(1):234-6.
19. Haviv M, Haver E, Lichtstein D, Hurvitz H, Klar A. Atrial natriuretic peptide in children with pneumonia. Pediatr Pulmonol. 2005;40(4):306-9.
20. Hartemink KJ, Groeneveld AB, de Groot MC, Strack van
Schijndel RJ, van Kamp G, Thijs LG. alpha-atrial natriuretic peptide, cyclic guanosine monophosphate, and endothelin in plasma as markers of myocardial depression in human septic shock. Crit Care Med. 2001;29(1):80-7.
21. Morgenthaler NG, Struck J, Christ-Crain M, Bergmann A, Müller B. Pro-atrial natriuretic peptide is a prognostic marker in sepsis, similar to the APACHE II score: an observational study. Crit Care. 2005;9(1):R37-45.
22. McDonagh TA, Robb SD, Murdoch DR, Morton JJ, Ford I, Morrison CE, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet. 1998;351(9095):9-13.
23. Itoi K, Jiang YQ, Iwasaki Y, Watson SJ. Regulatory mechanisms of corticotropin-releasing hormone and vasopressin gene expression in the hypothalamus. J Neuroendocrinol. 2004;16(4):348-55.
24. Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500-4.
25. Morgenthaler NG, Müller B, Struck J, Bergmann A, Redl H, Christ-Crain M. Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock. 2007;28
(2):219-26.
26. Müller B, Süess E, Schuetz P, Müller C, Bingisser R, Bergmann A, et al. Circulating levels of pro-atrial natriuretic peptide in lower respiratory tract infections. J Intern Med. 2006;260(6):568-76.
27. Masiá M, Papassotiriou J, Morgenthaler NG, Hernández I, Shum C, Gutiérrez F. Midregional pro-A-type natriuretic peptide and carboxy-terminal provasopressin may predict prognosis in community-acquired pneumonia. Clin Chem. 2007;53(12):2193-201.
28. Krüger S, Papassotiriou J, Marre R, Richter K, Schumann C, von Baum H, et al. Pro-atrial natriuretic peptide and pro-vasopressin to predict severity and prognosis in community-acquired pneumonia: results from the German competence network CAPNETZ. Intensive Care Med. 2007;33(12):2069-78.
29. Ugarte H, Silva E, Mercan D, De Mendonça A, Vincent JL. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999;27(3):498-504.
30. Yentis SM, Soni N, Sheldon J. C-reactive protein as an indicator of resolution of sepsis in the intensive care unit. Intensive Care Med. 1995;21(7):602-5.
* Study conducted at the Porto Alegre Hospital de Clínicas, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Correspondence to: Renato Seligman. Rua General Ibá M. I. Moreira, 199-8, CEP 91340-190, Porto Alegre, RS, Brasil.
Tel. 55 51 3332-6544; Fax: 55 51 3332-6454. E-mail: reseligman@hcpa.ufrgs.br
Financial support: This study received financial support from the Fundo de Incentivo à Pesquisa (FIPE, Research Incentive Fund) of the Hospital de Clínicas de Porto Alegre.
Submitted: 1 March 2011. Accepted, after review: 24 May 2011.
** A versão completa em português deste artigo está disponível em www.jornaldepneumologia.com.brOriginal
About the authorsRenato Seligman
Adjunct Professor. Department of Internal Medicine, Federal University of Rio Grande do Sul School of Medicine, Porto Alegre, Brazil.
Beatriz Graeff Santos Seligman
Adjunct Professor. Department of Internal Medicine, Federal University of Rio Grande do Sul School of Medicine, Porto Alegre, Brazil.
Paulo José Zimermann Teixeira
Adjunct Professor. Federal University of Health Sciences in Porto Alegre, Porto Alegre, Brazil.






