ABSTRACT
Objective: To analyze mortality from idiopathic pulmonary fibrosis (IPF) in Brazil over the period 1979-2014. Methods: Microdata were extracted from the Brazilian National Ministry of Health Mortality Database. Only deaths for which the underlying cause was coded as International Classification of Diseases version 9 (ICD-9) 515 or 516.3 (until 1995) or as ICD version 10 (ICD-10) J84.1 (from 1996 onward) were included in our analysis. Standardized mortality rates were calculated for the 2010 Brazilian population. The annual trend in mortality rates was analyzed by joinpoint regression. We calculated risk ratios (RRs) by age group, time period of death, and gender, using a person-years denominator. Results: A total of 32,092 deaths were recorded in the study period. Standardized mortality rates trended upward, rising from 0.24/100,000 population in 1979 to 1.10/100,000 population in 2014. The annual upward trend in mortality rates had two inflection points, in 1992 and 2008, separating three distinct time segments with an annual growth of 2.2%, 6.8%, and 2.4%, respectively. The comparison of RRs for the age groups, using the 50- to 54-year age group as a reference, and for the study period, using 1979-1984 as a reference, were 16.14 (14.44-16.36) and 6.71 (6.34-7.12), respectively. Men compared with women had higher standardized mortality rates (per 100,000 person-years) in all age groups. Conclusion: Brazilian IPF mortality rates are lower than those of other countries, suggesting underdiagnosis or underreporting. The temporal trend is similar to those reported in the literature and is not explained solely by population aging.
Keywords:
Idiopathic pulmonary fibrosis/epidemiology; Idiopathic pulmonary fibrosis/mortality; Population dynamics.
RESUMO
Objetivo: Analisar a mortalidade por fibrose pulmonar idiopática (FPI) no Brasil no período de 1979-2014. Métodos: Foram extraídos microdados do Sistema de Informações de Mortalidade do Ministério da Saúde cuja causa básica de óbito tenha sido codificada conforme a Classificação Internacional das Doenças, 9ª edição, códigos 515 ou 516.3 (até 1995), e 10ª versão, código J84.1 (a partir de 1996). Os coeficientes de mortalidade padronizados foram calculados para a população brasileira de 2010. A tendência anual da mortalidade foi analisada pelo método de regressão por pontos de inflexão. Foram calculadas as razões de risco (RR) por faixa etária, período analisado e gênero, utilizando-se como denominador pessoas-ano. Resultados: Foram registrados 32.092 óbitos no período estudado. O coeficiente de mortalidade padronizado mostrou-se ascendente, passando de 0,24/100.000 habitantes em 1979 para 1,10/100.000 em 2014. A tendência anual de crescimento identificou dois pontos de inflexão, em 1992 e 2008, gerando três retas com crescimento anual percentual de 2,2%, 6,8% e 2,4%, respectivamente. As RR (IC95%) por faixa etária, elegendo a faixa de 50-54 anos como referência, e por período estudado, elegendo o período de 1979-1984 como referência, foram de 16,14 (14,44-16,36) e de 6,71 (6,34-7,12), respectivamente. Homens, comparados a mulheres, apresentaram taxas de mortalidade padronizadas (por 100.000 pessoas-ano) superiores em todas as faixas etárias. Conclusões: Os coeficientes de mortalidade brasileiros por FPI são inferiores aos de outros países, podendo indicar subdiagnóstico ou subnotificação. A tendência temporal é semelhante à descrita na literatura e não é justificada apenas pelo envelhecimento populacional.
Palavras-chave:
Fibrose pulmonar idiopática/epidemiologia; Fibrose pulmonar idiopática/ mortalidade; Dinâmica populacional.
INTRODUCTIONIdiopathic pulmonary fibrosis (IPF) is a disease of unknown cause, predominantly occurring in individuals over 60 years of age and usually exhibiting rapid clinical progression and high lethality, with a median survival of less than 5 years.(1) Estimates of prevalence and incidence vary according to the data sources used and to the broadness or narrowness of the criteria for disease definition.(2-4) It is known that mortality from IPF has been increasing worldwide, ranging from 4 to 10 deaths per 100,000 population in ten countries in Europe, North America, Asia, and Oceania.(5)
Studying the rates of occurrence of IPF is an aiding tool in developing appropriate health policies and in planning IPF management.(4) Recently, the availability of antifibrotic drugs with the potential to slow down the functional decline associated with IPF has sharpened the need for precision, on the part of specialists, in identifying the disease, given the possibility of a change in the clinical approach.(6,7) Because the symptoms and imaging findings of IPF have similarities with those of other interstitial lung diseases, added weight has been given to the differential diagnosis, since other diseases of similar presentation progress differently, require different approaches, and differ in prognosis.(8)
National data reveal that, in the Brazilian state of Rio Grande do Sul, mortality from IPF increased significantly between 1970 and 2000, rising from 0.22/100,000 population to 0.48/100,000 population during that period. (9) In Brazil, another analysis of mortality showed that there was an increase from 0.65/100,000 deaths in 1996 to 1.21/100,000 deaths in 2010.(10)
The objective of the present study was to investigate mortality from IPF as the underlying cause and examine its temporal trend, on the basis of deaths occurring between 1979 and 2014 in Brazil.
METHODSStudy designThis was an ecological study of temporal trends in mortality from IPF as the underlying cause, involving analysis of aggregate data from the Brazilian National Ministry of Health Sistema de Informações de Mortalidade (SIM, Mortality Database), for the period 1979 to 2014. Because of possible inclusion of inaccurate data in the SIM (deaths from other interstitial lung diseases, here referred to as "similars"), cases were defined as deaths for which the underlying cause was attributed to IPF + similars (IPF-S).(11)
Microdata were extracted from the SIM, in an anonymized fashion, for the period January 1, 1979 to December 31, 2014. During that period, two versions of the International Classification of Diseases (ICD) were used: ICD version 9 (ICD-9), from 1979 to 1995; and ICD version 10 (ICD-10), from 1996 onward. Deaths occurring until December 31, 1995 for which the underlying cause was coded as ICD-9 515 (post-inflammatory pulmonary fibrosis) or 516.3 (idiopathic pulmonary fibrosis) in the SIM were included in our analysis. For deaths occurring from January 1, 1996 onward, code J84.1 (other interstitial lung diseases with fibrosis) was used, assuming that the diseases were equivalent to IPF.(12) For each unit of observation, information on year of death, age, and gender was collected.
Data analysisAnnual crude mortality rates were calculated using the population of each year, obtained through census data for years 1980, 1991, 2000, and 2010 or from estimates for intercensal years provided by the Brazilian Institute of Geography and Statistics. The annual crude mortality rates were adjusted to the 2010 census in order to obtain standardized mortality rates per 100,000 population.
The annual percent change (APC) in the rates was calculated using joinpoint regression(13) (Joinpoint Regression Program; Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program, National Cancer Institute, Rockville, MD, USA). The inflection points indicate statistically significant changes in the curve.
Mortality was also analyzed by obtaining standardized mortality rates per 100,000 person-years, which were calculated for three variables: a) age-first age group: 0-49 years, followed by groups covering a 5-year age range; b) time-~5-year time periods of death, with no combination of the period preceding and that following the ICD change; and c) gender. For calculating person-years, the 2010 Brazilian population was used as a reference. For age, the number of person-years was estimated by multiplying the population within each age group by the midpoint of that range. Confidence intervals were calculated assuming a Poisson distribution and using Byar approximation. Risk ratios (RRs) were calculated using the 50- to 54-year age group, the first time period studied (1979-1984), and the male gender as a reference. The corresponding confidence intervals were calculated using Taylor series. These calculations were performed using OpenEpi, version 3.01.
Ethical aspectsBecause the present study used anonymized secondary public domain data, available, with unrestricted access, in government digital databases, it did not require approval from an ethics committee.
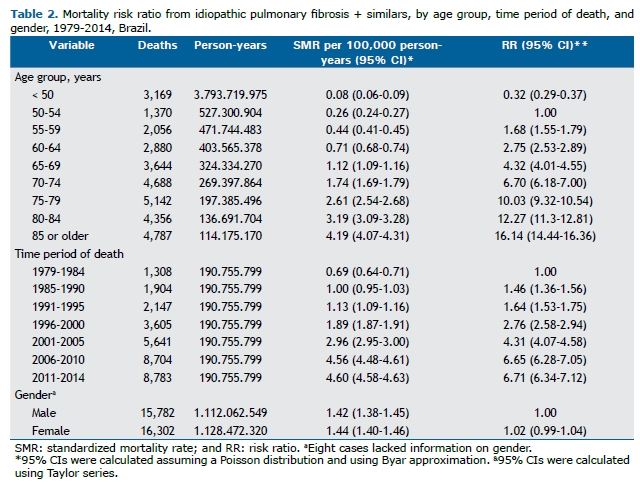
RESULTSBetween 1979 and 2014, there were 32,092 deaths for which the underlying cause was IPF-S. Of those, 3,169 (9.9%) were deaths of individuals up to 49 years of age. Regarding gender, there were 15,782 and 16,302 deaths of men and women, respectively. In 8 cases, there was no information about gender. Among individuals 75 years of age or older, the absolute number of deaths of women was greater than that of men.
Crude and standardized mortality rates trended upward, with the latter rising from 0.24/100,000 population in 1979 to 1.10/100,000 population in 2014. Figure 1 shows the temporal variation over the study period. There were no differences in the pattern of increase in mortality rates when women and men were analyzed separately.
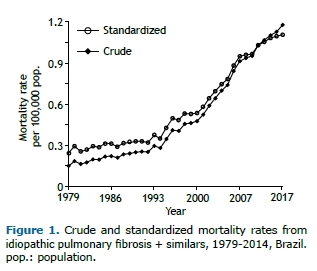
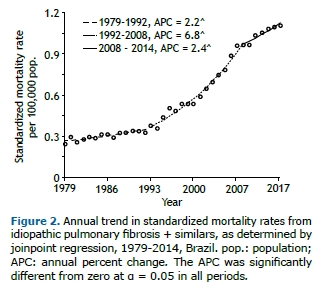
The temporal trend in standardized mortality rates had two inflection points separating three distinct time segments: 1979-1992; 1992-2008; and 2008-2014. Figure 2 shows the time segments and their corresponding APCs. There was a significant increase in mortality throughout the time period analyzed; however, in the years 1992 to 2008, there was an average annual growth of 6.8%.
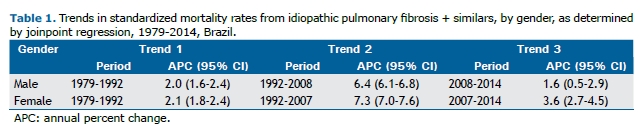
The annual increase in APCs differed slightly between genders, and the trends for both genders had two inflection points (Table 1). The greatest increase in the rates occurred between 1992 and 2008 in men and between 1992 and 2007 in women.
Analysis of census population changes occurring between 1980 and 2010, during which time there were four demographic censuses, revealed a 68% increase in the population 50 years of age or older, which increased by 61% among men and by 74% among women. In that same period, the increase in standardized mortality rates from IPF-S among individuals 50 years of age or older was 317%.
The RRs, calculated from mortality rates per person-years, are shown in Table 2. There was a strong upward trend in mortality as the age group increased and by time period of death. The rates, adjusted by person-years, showed that mortality increased by a factor of more than 16 when comparing the age groups, using the 50- to 54-year age group as a reference, and by a factor of more than 6, when comparing the time periods of death, using the first time period studied as a reference. There were no gender-related differences in overall standardized mortality rates (95% CI: 0.99-1.04).
Analysis by gender and age group showed that the standardized mortality rates per 100,000 person-years were higher in men, with the differences in RR between men and women being statistically significant in all age groups, except in the 85-year or older age group (Figure 3).
 DISCUSSION
DISCUSSIONThe data obtained from the Brazilian National Ministry of Health SIM showed a consistent increase in mortality from IPF-S as the underlying cause over the period 1979-2014. During that period, the standardized mortality rates increased 4.6 times, rising from 0.24 to 1.10 per 100,000 population. The upward trend in mortality from IPF had been described in the Brazilian state of Rio Grande do Sul for the period 1970-2000(9) and in Brazil for the period 1996-2010.(10) Possibly, the small difference between the study by Rufino et al.(10) and ours is due to the reference population used for standardization of rates. In the present study, we did not include any mention of IPF on other lines of the death certificate because this information was unavailable in the SIM until 1999.
In our study, analysis of the annual trend in mortality revealed a marked annual increase, with the average annual growth being strongest between 1992 and 2008 (6.8%). In comparison, an analysis of mortality in ten countries revealed an average annual growth of 2-3% between 1999 and 2012.(5) The change from ICD-9 to ICD-10 in 1996 was not accompanied by a change in curve inflection, which demonstrates that there was no detectable impact on the reporting of deaths.
The increase in mortality rates, as well as the existence of a period when growth was most marked (1992-2008), is possibly due to a combination of factors. Technical issues that are hard to "measure", such as the increased availability and use of HRCT after the 1990s, as well as the advances in CT scanner technology, have made it possible to define, with increasing precision, the imaging aspects associated with IPF.(14,15) Additionally, the practice of multidisciplinary discussions among medical specialties involved in the investigation of interstitial lung diseases has been adopted, which has facilitated the differential diagnosis of this group of diseases, possibly preventing misclassifications.(16)
Demographic factors have also contributed to explaining the increase in mortality rates. According to census counts, between 1980 and 2010, the Brazilian population over 50 years of age increased by 68%. The standardized mortality rates per person-years ranged from 0.69/100,000 population in the first period (1979-1984) to 4.60/100,000 population in the last period (2011-2014); therefore, there was an almost sevenfold increase between the first and last observation periods. In comparison, in England and Wales, an analysis of mortality from IPF-S as the underlying cause revealed a sixfold increase between the first and last observation periods (total observation period: 1968 to 2008).(11) Although the period analyzed in that study does not exactly correspond to that of the present study, the greater increase observed here could be partially explained by the marked change in Brazil's population pyramid relative to that of those countries.
The standardized mortality rates per person-years were higher in men in all age groups. However, the male-to-female death ratio was 0.97, in comparison with 1.57 in the study of mortality in England and Wales(11) and with 1.29 in the study of the incidence of IPF in Canada.(4) A likely explanation for this finding is the marked difference in life expectancy between men and women in Brazil compared with industrialized countries: over the period 2005-2010, the difference in life expectancy between men and women was close to 4 years in Great Britain and in Canada, whereas it was 8 years in Brazil.(17) The numerical excess of deaths from IPF-S in women is essentially due to greater female survival and to the greater number of females in the general population. Except in the 85-year or older age group, all RRs were significantly higher in men. It should be noted that, in three referral centers in Brazil, the extent to which the proportion of individuals with IPF was higher among men than women was more pronounced than that found in the present study.(18)
Problems in comparing statistics on IPF lie in the data extraction source and in the criteria for defining the disease.(2-4) However, there is a consensus that the incidence of IPF has been gradually rising, approaching that of diseases such as stomach and liver cancer, as well as cervical cancer.(5,19) The nationwide study in Canada revealed a higher incidence of IPF in the more industrialized areas of the country.(4) In the literature, there are reports of significantly increased risk of IPF for the following occupational and environmental factors: working in agriculture; raising livestock; working with wood; being exposed to metal dusts; being exposed to dusts containing silica; and smoking.(20) This indicates the need to explore possible occupational and environmental exposures. Recently, it has been demonstrated that deaths from IPF-S and from mesothelioma show a significant linear relationship with historical data on asbestos imports in Great Britain.(21) It should be pointed out that, because of its clinical presentation and, especially, its imaging presentation, asbestosis is one of the main differential diagnoses of IPF.(22) A review of 1,718 cases of lobectomy for lung tumors demonstrated that, among patients with asbestos exposure markers, the proportion of histology results consistent with usual interstitial pneumonia was significantly higher than it was among patients without these markers, it being concluded that there is evidence that asbestos exposure leads to the development of IPF.(23) Given the long-term, historically high consumption of asbestos in Brazil, this merits attention, because the legal and clinical management implications of asbestosis are very different from those of IPF.
Population-based mortality data have the advantages of the strength of large numbers, of not requiring extrapolations, and of making it possible to analyze long historical series. Because of the high lethality of IPF, mortality and incidence rates are close. However, an important limitation associated with the use of mortality data lies in the reliability of the diagnosis. In the present analysis, it was not possible to review the clinical data on the recorded deaths. It is likely that other interstitial lung diseases have been erroneously coded as ICD-9 515, ICD-9 516.3, or as ICD-10 J84.1, which is why we adopted the term IPF-S, similarly to the study by Navaratnam et al.(11) Differences in utilization of ICD codes by physicians who certify deaths and the lack of a specific ICD code for IPF preclude the determination of the actual mortality rate.(2) Another important limitation concerns the completeness of mortality data in the SIM. From 2000 onward, the estimated coverage of the SIM was 100% for the southern region and nearly 100% for the southeastern region. However, for the northern region, in 2000 and 2010, the estimated coverage was 75.3% and 85.4%, respectively.(12) Given the heterogeneity in the completeness of mortality data in Brazil, it is likely that there are regional differences in mortality from IPF-S.
Although IPF is highly lethal, underlying cause-of-death data alone could mask the actual incidence of the disease. In England and Wales, it was estimated that approximately 60% of patients with known IPF had this disease recorded as the underlying cause of death on the death certificate.(24) Despite its limitations, the use of underlying cause-of-death data revealed an upward temporal trend similar to that obtained when analyzing IPF cases derived from outpatient clinical records of chronic disease patients, which are records that have higher diagnostic accuracy.(11)
We conclude that mortality from IPF-S in Brazil shows an upward trend, similar to that reported in other countries, but with low mortality rates, suggesting that the disease is underdiagnosed and/or underreported. The change in Brazil's population pyramid does not alone explain the observed increase. Other studies with appropriate designs should be conducted to provide a better understanding of the findings.
ACKNOWLEDGMENTSWe are grateful to Dr. Maria Paula Curado and the JBP reviewers for their critical reading of the manuscript and their suggestions.
REFERENCES1. Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431-40. https://doi.org/10.1164/rccm.201006-0894CI
2. Samet JM, Coultas D, Raghu G. Idiopathic pulmonary fibrosis: tracking the true occurrence is challenging. Eur Respir J. 2015;46(3):604-6. https://doi.org/10.1183/13993003.00958-2015
3. Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810-6. https://doi.org/10.1164/rccm.200602-163OC
4. Hopkins RB, Burke N, Fell C, Dion G, Kolb M. Epidemiology and survival of idiopathic pulmonary fibrosis from national data in Canada. Eur Respir J. 2016;48(1):187-95. https://doi.org/10.1183/13993003.01504-2015
5. Hutchinson JP, McKeever TM, Fogarty AW, Navaratnam V, Hubbard RB. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann Am Thorac Soc. 2014;11(8):1176-85. https://doi.org/10.1513/AnnalsATS.201404-145OC
6. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-82. https://doi.org/10.1056/NEJMoa1402584
7. King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083-92. https://doi.org/10.1056/NEJMoa1402582
8. Martinez FJ, Chisholm A, Collard HR, Flaherty KR, Myers J, Raghu G, et al. The diagnosis of idiopathic pulmonary fibrosis: current and future approaches. Lancet Respir Med. 2017;5(1):61-71. https://doi.org/10.1016/S2213-2600(16)30325-3
9. Fortuna FP, Perin C, Cunha L, Rubin AS. Mortality due to idiopathic pulmonary fibrosis in the State of Rio Grande do Sul (Brazil). J Pneumol. 2003;29(3):1-4. https://doi.org/10.1590/S0102-35862003000300002
10. Rufino RL, Costa CH, Accar J, Torres GR, Silva VL, Barros NP, et al. Incidence and mortality of interstitial pulmonary fibrosis in Brazil. Am J Respir Crit Care Med. 2013;187:A1458
11. Navaratnam V, Fleming KM, West J, Smith CJ, Jenkins RG, Fogarty A, et al. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax. 2011;66(6):462-7. https://doi.org/10.1136/thx.2010.148031
12. Brasil. Ministério da Saúde. Rede Interagencial de Informações para a Saúde [homepage on the Internet]. Brasília: o Ministério; [updated 2013 Dec 20; cited 2016 Jul 20]. Indicadores e Dados Básicos - Brasil - 2012. Available from: http://tabnet.datasus.gov.br/cgi/idb2012/matriz.htm#cobe
13. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335-51. https://doi.org/10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z
14. Mathieson JR, Mayo JR, Staples CA, Müller NL. Chronic diffuse infiltrative lung disease: comparison of diagnostic accuracy of CT and chest radiography. Radiology. 1989;171(1):111-6. https://doi.org/10.1148/radiology.171.1.2928513
15. Swensen SJ, Aughenbaugh GL, Myers JL. Diffuse lung disease: diagnostic accuracy of CT in patients undergoing surgical biopsy of the lung. Radiology. 1997;205(1):229-34. https://doi.org/10.1148/radiology.205.1.9314990
16. Hunninghake GW, Zimmerman MB, Schwartz DA, King TE Jr, Lynch J, Hegele R, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(2):193-6. https://doi.org/10.1164/ajrccm.164.2.2101090
17. United Nations. Department of Economic and Social Affairs. Population Division (2015). World Population Prospects. The 2015 Revision. Volume I: Comprehensive Tables. ST/ESA/SER.A/379
18. Soares MR, Pereira C, Ferreira R, Nei Aparecida Martins Coletta E, Silva Lima M, Muller Storrer K. A score for estimating survival in idiopathic pulmonary fibrosis with rest SpO2>88. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32(2):121-8
19. Hutchinson J, Fogarty A, Hubbard R, McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46(3):795-806. https://doi.org/10.1183/09031936.00185114
20. Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc. 2006;3(4):293-8. https://doi.org/10.1513/pats.200512-131TK
21. Barber CM, Wiggans RE, Young C, Fishwick D. UK asbestos imports and mortality due to idiopathic pulmonary fibrosis. Occup Med (Lond). 2016;66(2):106-11. https://doi.org/10.1093/occmed/kqv142
22. Valeyre D, Jeny F, Freynet O, Nunes H. Key diagnostic issues. In: Idiopathic Pulmonary Fibrosis 2016. ERS Monogr. 2016;71:50-56. https://doi.org/10.1183/2312508X.10004915
23. Kawabata Y, Shimizu Y, Hoshi E, Murai K, Kanauchi T, Kurashima K, et al. Asbestos exposure increases the incidence of histologically confirmed usual interstitial pneumonia. Histopathology. 2016;68(3):339-46. https://doi.org/10.1111/his.12751
24. Johnston I, Britton J, Kinnear W, Logan R. Rising mortality from cryptogenic fibrosing alveolitis. BMJ. 1990;301(6759):1017-21. https://doi.org/10.1136/bmj.301.6759.1017








