ABSTRACT
Objective: Smoking prevalence is frequently estimated on the basis of self-reported smoking status. That can lead to an underestimation of smoking rates. The aim of this study was to evaluate the difference between self-reported smoking status and that determined through the use of objective measures of smoking at a pulmonary outpatient clinic. Methods: This was a cross-sectional study involving 144 individuals: 51 asthma patients, 53 COPD patients, 20 current smokers, and 20 never-smokers. Smoking status was determined on the basis of self-reports obtained in interviews, as well as through tests of exhaled carbon monoxide (eCO) and urinary cotinine. Results: All of the asthma patients and COPD patients declared they were not current smokers. In the COPD and asthma patients, the median urinary cotinine concentration was 167 ng/mL (range, 2-5,348 ng/mL) and 47 ng/mL (range, 5-2,735 ng/mL), respectively (p < 0.0001), whereas the median eCO level was 8 ppm (range, 0-31 ppm) and 5 ppm (range, 2-45 ppm), respectively (p < 0.05). In 40 (38%) of the patients with asthma or COPD (n = 104), there was disagreement between the self-reported smoking status and that determined on the basis of the urinary cotinine concentration, a concentration > 200 ng/mL being considered indicative of current smoking. In 48 (46%) of those 104 patients, the self-reported non-smoking status was refuted by an eCO level > 6 ppm, which is also considered indicative of current smoking. In 30 (29%) of the patients with asthma or COPD, the urinary cotinine concentration and the eCO level both belied the patient claims of not being current smokers. Conclusions: Our findings suggest that high proportions of smoking pulmonary patients with lung disease falsely declare themselves to be nonsmokers. The accurate classification of smoking status is pivotal to the treatment of lung diseases. Objective measures of smoking could be helpful in improving clinical management and counseling.
Keywords:
Asthma; Pulmonary disease, chronic obstructive; Cotinine; Carbon monoxide; Smoking.
RESUMO
Objetivo: O tabagismo autodeclarado é usado frequentemente para estimar a prevalência dessa condição. As taxas de tabagismo podem ser subestimadas por esse método. O objetivo deste estudo foi avaliar a diferença entre o tabagismo autodeclarado e o tabagismo determinado pelo uso de medidas objetivas em um ambulatório de doenças respiratórias. Métodos: Estudo transversal realizado em 144 indivíduos: 51 pacientes com asma, 53 pacientes com DPOC, 20 fumantes e 20 não fumantes. O tabagismo foi determinado por meio de autorrelato em entrevistas e medição de monóxido de carbono no ar exalado (COex) e de cotinina urinária. Resultados: Todos os pacientes com asma e DPOC declararam não ser fumantes. Nos pacientes com DPOC e asma, a mediana de concentração de cotinina urinária foi de 167 ng/ml (variação, 2-5.348) e de 47 ng/ml (variação, 5-2.735 ppm), respectivamente (p < 0,0001), enquanto . a mediana de COex foi de 8 ppm (variação, 0-31) e 5,0 ppm (variação, 2-45 ppm), respectivamente (p < 0,05). Em 40 (38%) dos pacientes com asma ou DPOC (n = 104), houve discordâncias entre o tabagismo autodeclarado e a concentração de cotinina urinária (> 200 ng/mL). Em 48 (46%) desses 104 pacientes, o não tabagismo autodeclarado foi refutado por um nível de COex > 6 ppm, considerado indicativo de fumo atual. Em 30 (29%) dos pacientes com asma ou DPOC, a concentração de cotinina urinária e o nível de COex contradisseram o autorrelato desses como não fumantes. Conclusões: Nossos achados sugerem que altas proporções de pacientes fumantes com doenças respiratórias declaram ser não fumantes. A classificação correta do tabagismo é fundamental no tratamento dessas doenças. Medidas objetivas do tabagismo podem ser úteis na melhora do manejo clínico e no aconselhamento.
Palavras-chave:
Asma; Doença pulmonar obstrutiva crônica; Cotinina; Monóxido de carbono; Hábito de fumar.
IntroduçãoO tabagismo, o principal fator de risco de DPOC,(1) pode agravar a inflamação associada à asma, fazendo com que os sintomas sejam mais graves, acelerando o declínio da função pulmonar e prejudicando a resposta terapêutica em curto prazo aos corticosteroides.(2) Embora autorrelatos de tabagismo sejam amplamente usados para estimar a prevalência de tabagismo em pacientes com asma ou DPOC,(3-5) demonstrou-se que seu uso subestima as taxas de tabagismo, especialmente em virtude da aceitabilidade social cada vez menor do tabagismo.(6) Alguns autores questionaram a validade do tabagismo autodeclarado na população em geral e relataram taxas significativas de classificação incorreta.(7)
A prevalência de tabagismo na população adulta na cidade de São Paulo (SP) em 2008 foi de 20,9%.(8) A avaliação do tabagismo é fundamental para o tratamento de doenças respiratórias. A cessação do tabagismo não só é considerada a intervenção mais eficiente para retardar a progressão da DPOC(9) como também pode melhorar o tratamento e a resposta ao tratamento em pacientes com asma.(2)
Em estudos de intervenção para a cessação do tabagismo, o uso de uma medida bioquímica foi considerado essencial para validar o tabagismo autodeclarado.(10) A determinação da concentração de monóxido de carbono no ar expirado (COex) é um método rápido e não invasivo de avaliação do tabagismo. Embora o CO tenha uma meia-vida de aproximadamente 4 h e seja detectável no sangue durante um período de até 24 h, a contribuição de fontes ambientais não pode ser distinguida da contribuição do tabagismo, o que pode levar a resultados positivos falsos. (11) Vários estudos demonstraram que valores de corte entre 6 e 8 ppm são apropriados para separar fumantes de não fumantes.(12) Se um indivíduo fuma apenas alguns cigarros por dia ou não fuma um cigarro há várias horas, o teste do COex pode produzir resultados falso negativos.(13) O principal metabólito da nicotina é a cotinina, e a cotinina urinária é um marcador específico da nicotina. Exceto em usuários de terapia de reposição de nicotina, concentrações elevadas de cotinina indicam uso de tabaco ou exposição à fumaça ambiental do tabaco.(14) As concentrações de cotinina são menos dependentes do tempo decorrido desde o último cigarro fumado do que o são os níveis de COex, pois a meia-vida da cotinina na urina é de aproximadamente 16 h.(15) No entanto, a concentração de cotinina urinária é altamente dependente do método de ensaio e do laboratório que realiza a análise, o que torna difícil a identificação de uma concentração de corte universal para classificar um indivíduo em fumante ou não fumante.(16)
O objetivo deste estudo foi comparar autorrelatos de tabagismo com medidas objetivas de tabagismo (cotinina urinária e COex) em pacientes com asma ou DPOC estável.
MétodosDesenho do estudoTrata-se de um estudo transversal envolvendo pacientes com asma ou DPOC recrutados entre aqueles tratados regularmente no Ambulatório de Pneumologia do Instituto do Coração, Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, em São Paulo (SP). Informações sobre o hábito de fumar, sintomas, estilo de vida, exposição e uso de medicamentos foram coletadas por um entrevistador. Todos os entrevistadores foram treinados para evitar pressionar ou julgar os pacientes. Todos os participantes receberam a garantia de que os resultados seriam confidenciais, a fim de incentivar relatos precisos de seus hábitos de fumar. O Comitê de Ética em Pesquisa do Hospital das Clínicas aprovou o protocolo do estudo, e todos os participantes assinaram um termo de consentimento livre e esclarecido.
ParticipantesOs diagnósticos de DPOC e asma foram baseados nas definições contidas nas diretrizes da Global Initiative for Chronic Obstructive Lung Disease(1) e da Global Initiative for Asthma,(17) respectivamente. Os pacientes com asma ou DPOC foram recrutados pessoalmente por membros da equipe de pesquisa ou entrevistadores após visitas regulares ao ambulatório. Os critérios de inclusão foram tratamento ambulatorial há pelo menos 12 meses no momento do recrutamento e ausência de alterações no regime de tratamento nas últimas 4 semanas. Os pacientes que estavam usando terapia de reposição de nicotina foram excluídos, assim como o foram aqueles com distúrbios cognitivos que teriam prejudicado sua capacidade de preencher um questionário, aqueles com insuficiência renal com necessidade de diálise e aqueles com deformidades faciais que teriam impedido o uso de espirometria ou a medição da concentração de COex. Para garantir que os resultados relativos à cotinina urinária e ao COex fossem confiáveis, foram também recrutados indivíduos normais sem asma, DPOC ou outros problemas respiratórios identificáveis: 20 fumantes (grupo controle positivo) e 20 indivíduos que nunca fumaram (grupo controle negativo). Os controles foram recrutados entre alunos e funcionários da universidade, por meio de cartazes afixados no hospital e na universidade. Foram usadas as seguintes definições de tabagismo: foram considerados fumantes os indivíduos que referiram uso atual e regular de cigarros; foram considerados indivíduos que nunca fumaram aqueles que referiram nunca ter fumado cigarros; foram considerados ex-fumantes os indivíduos que referiram ter fumado 100 ou mais cigarros na vida e que, no momento da inclusão no estudo, estivessem abstinentes há no mínimo 12 meses.
Determinação do tabagismo autodeclaradoImediatamente após o recrutamento, foram realizadas entrevistas cara a cara para coletar dados relativos à história de saúde e características demográficas. Os participantes responderam às seguintes perguntas: "Você fuma?"; "Você está fumando agora?"; "Quando você parou de fumar?"; "Quantos cigarros você fuma por dia?"; "Quantos fumantes moram em seu domicílio?". As respostas a essas perguntas foram registradas em um fluxograma como dados nominais (sim/não) ou dados de intervalo.
Testes de função pulmonarEm todos os participantes, o VEF1 e a CVF foram medidos com um espirômetro (KoKo; nSpire Health Inc., Longmount, CO, EUA). Todos os procedimentos de espirometria foram realizados de acordo com as recomendações conjuntas da American Thoracic Society e da European Respiratory Society.(18) Todos os testes de função pulmonar foram realizados entre 8h00 e 12h00.
Determinação da concentração de cotinina urináriaPara determinar as concentrações de cotinina urinária, foram coletadas amostras de urina matinal no momento de uma consulta no ambulatório. As amostras de urina foram coletadas em frascos esterilizados. Alíquotas dessas amostras foram armazenadas a −80°C para posterior análise laboratorial em lotes.
A análise quantitativa da cotinina nas amostras de urina foi realizada por meio de um método modificado de HPLC. Uma concentração de cotinina > 200 ng/ml é considerada indicativa de uso ativo de produtos contendo nicotina.(19,20)
Determinação da concentração de COexOs níveis de COex foram medidos em uma amostra de ar expirado com um testador de CO (Micro CO; Micro Medical Ltd., Rochester, Reino Unido). Os participantes receberam uma explicação detalhada a respeito do teste de análise do ar expirado e tiveram a oportunidade de praticar. Embora apresente boa reprodutibilidade,(21) o teste foi realizado em duplicata para garantir a consistência. Os valores de COex foram expressos em ppm; valores 0-6 ppm indicam ausência de tabagismo, ao passo que valores > 6 ppm sugerem a presença de tabagismo.(13,22) Antes de cada teste, os níveis ambientais de CO foram registrados com o testador de CO calibrado com ar ambiente por meio de uma seringa de calibração. Os testes de COex foram realizados entre 8h00 e 12h00.
Cálculo do tamanho da amostraO tamanho da amostra foi calculado com o objetivo de selecionar uma amostra que fosse suficiente para detectar uma diferença de 10% entre o tabagismo autodeclarado e o tabagismo detectado por meio de medição objetiva. Assim, determinou-se que seria necessária uma amostra de cerca de 140 indivíduos de modo a atingir um poder de 80% com significância bicaudal de 0,05.
Análise estatísticaAs variáveis contínuas são apresentadas em forma de média erro-padrão; dados não paramétricos são apresentados em forma de mediana (intervalo interquartil) e as variáveis categóricas são apresentadas em forma de frequências absolutas e relativas. Testes do qui-quadrado foram usados para avaliar qualquer discordância entre o tabagismo autodeclarado e o tabagismo determinado por meio de medidas objetivas. Para comparar as características dos pacientes em função do tabagismo, da concentração de COex e da concentração de cotinina urinária, foram usados testes t de Student, o teste de Mann-Whitney e ANOVA com um fator. Para avaliar a força das associações entre variáveis contínuas relacionadas com as características dos pacientes, foi calculado o coeficiente de correlação de Pearson. Por fim, foi usada a análise de regressão logística do tipo stepwise para comparar pacientes classificados incorretamente a pacientes que forneceram informações confiáveis sobre seu hábito de fumar, com o objetivo de identificar preditores de tais erros de classificação. As características incluídas nessas análises foram idade, escolaridade e função pulmonar. Foi adotado um nível mínimo de significância de 0,05. Todas as análises estatísticas foram realizadas com o programa SigmaStat, versão 3.5 (Systat Software Inc., San Jose, CA, EUA).
ResultadosO fluxograma de recrutamento é apresentado na Figura 1. Dos 213 indivíduos aptos para participar do estudo, 69 foram excluídos da análise (por não terem preenchido os critérios do estudo, por não terem dado consentimento, por não observarem o protocolo ou por outros motivos). Portanto, a amostra final do estudo compreendeu um total de 144 participantes (70 homens e 74 mulheres): 53 pacientes com DPOC (37 homens e 16 mulheres); 51 pacientes com asma (16 homens e 35 mulheres); 20 fumantes (9 homens e 11 mulheres) e 20 indivíduos que nunca fumaram (8 homens e 12 mulheres). Nenhum dos indivíduos incluídos no estudo estava usando terapia de reposição de nicotina durante a avaliação. Todos os pacientes com asma ou DPOC declararam não ser fumantes. Declararam ser ex-fumantes 51 pacientes com DPOC e 12 pacientes com asma, os quais afirmaram que haviam parado de fumar 1-17 anos atrás. Relataram que compartilhavam um domicílio com um ou mais fumantes (mediana de um fumante em cada um dos dois grupos) 28 pacientes com DPOC e 15 pacientes com asma. Tanto os pacientes com asma como os com DPOC apresentaram função pulmonar prejudicada, com média de VEF1 de 57% e 36% do valor previsto, respectivamente. Isso indica que os pacientes de nossa amostra apresentavam as formas graves de suas respectivas doenças. Os dados clínicos e funcionais são apresentados na Tabela 1.

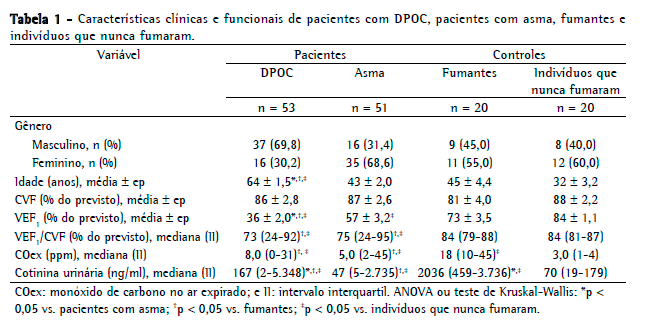
Como se pode observar na Figura 2, a mediana da concentração de COex nos indivíduos que nunca fumaram e nos fumantes foi de 3,0 ppm (variação: 1-4 ppm) e 18 ppm (variação: 10-45 ppm), respectivamente (p < 0,05), e de 8,0 ppm (variação: 0-31 ppm) e 5,0 ppm (variação: 2-45 ppm), respectivamente, nos pacientes com DPOC e nos pacientes com asma (p < 0,05). As concentrações de CO no ar ambiente foram de 0-2 ppm durante as medições.

A Figura 3 mostra as concentrações de cotinina urinária. A mediana da concentração de cotinina urinária foi de 70 ng/ml (variação: 19-179 ng/ml) nos indivíduos que nunca fumaram e de 2.036 ng/ml (variação: 459-3.736 ng/ml) nos fumantes, respectivamente (p < 0,05). A mediana da concentração de cotinina urinária foi de 167 ng/ml (variação: 2-5.348 ng/ml) nos pacientes com DPOC e de 47 ng/ml (variação: 5-2.735 ng/ml) nos pacientes com asma (p < 0,05).

Todos os 20 fumantes do grupo controle positivo apresentaram resultados positivos nos testes de tabagismo, com concentrações de cotinina urinária > 200 ng/ml e níveis de COex > 6 ppm. Por outro lado, todos os 20 indivíduos do grupo controle negativo (que nunca fumaram) apresentaram resultados negativos nos dois testes.
As concentrações de cotinina urinária foram > 200 ng/ml em 15 pacientes com asma (29%) e em 25 pacientes com DPOC (47%). Além disso, foram registrados níveis de COex > 6 ppm em 16 pacientes com asma (31%) e em 32 pacientes com DPOC (60%). Portanto, os resultados dos ensaios de cotinina urinária e dos testes de COex, respectivamente, sugeriram que 40 (38%) e 48 (46%) dos 104 pacientes foram incorretamente classificados em não fumantes com base em seus autorrelatos. A combinação de COex > 6 ppm com cotinina urinária > 200 ng/ml foi identificada em 7 pacientes com asma (14%) e em 23 pacientes com DPOC (43%), correspondendo coletivamente a 29% do total de pacientes da amostra.
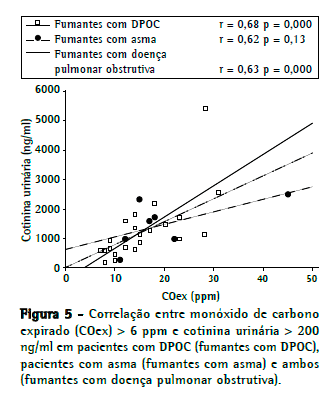
Como se pode observar na Figura 4, a análise univariada mostrou que a concentração de COex correlacionou-se com a concentração de cotinina urinária: total (r = 0,43, p = 0,05); em pacientes com asma (r = 0,57, p < 0,0001) e em pacientes com DPOC (r = 0,69, p < 0,0001). A análise isolada dos pacientes que foram identificados como fumantes (Figura 5) revelou que uma concentração de COex > 6 ppm correlacionou-se significativamente com uma concentração de cotinina urinária > 200 ng/ml nos pacientes com DPOC (r = 0,68, p < 0,0003), mas não nos pacientes com asma (r = 0,62, p = 0,13). A análise coletiva dos pacientes com asma e dos pacientes com DPOC identificados como fumantes (Figura 5) revelou que ainda havia uma forte correlação entre uma concentração de COex > 6 ppm e uma concentração de cotinina urinária > 200 ng/mL (r = 0,63, p < 0,0001). A regressão logística do tipo stepwise, ajustada de acordo com características como idade, escolaridade, tabagismo passivo e função pulmonar, não identificou preditores de classificação incorreta de tabagismo.

 Discussão
DiscussãoOs resultados do presente estudo sugerem que pacientes com asma ou DPOC comumente fornecem informações incorretas sobre seu hábito de fumar. Embora esse comportamento tenha sido mais evidente entre os pacientes com DPOC, os pacientes com asma também subnotificaram o hábito de fumar. Nosso estudo mostrou também que a medição do COex identifica a maioria dos fumantes, e que o COex correlaciona-se significativamente com a cotinina urinária. Vale ressaltar que a correlação entre COex e cotinina urinária não foi estatisticamente significativa nos pacientes com asma (p = 0,13). É possível que isso tenha ocorrido em virtude de falta de poder (erro tipo II), já que poucos pacientes com asma apresentaram resultado positivo no teste de cotinina urinária. Quando os pacientes com DPOC e os pacientes com asma foram avaliados como um grupo, a correlação foi significativa. Foram poucos os estudos que exploraram a associação entre doenças pulmonares obstrutivas e tabagismo por meio da altamente sensível e específica medição da cotinina urinária e do COex para a validação bioquímica.
Como o tabagismo é crítico para o tratamento clínico da DPOC e da asma, é surpreendente que haja tamanha escassez de estudos sobre a invalidade dos relatos de pacientes com doenças pulmonares obstrutivas que declaram não ser fumantes embora sejam fumantes "de verdade". No presente estudo, verificou-se que, embora todos os pacientes tenham declarado não ser fumantes, 38% (47% dos pacientes com DPOC e 29% dos pacientes com asma) apresentaram concentrações de cotinina urinária > 200 ng/ml, um valor fortemente relacionado com tabagismo ativo.(20)
Um estudo realizado na Espanha mostrou que 17% de todos os pacientes tratados em uma clínica de medicina respiratória continuavam a fumar, embora negassem que o fizessem; uma proporção mais elevada (34%) foi observada nos pacientes com DPOC.(23) Em outro estudo, realizado na França, a medição de cotinina em pacientes que recebiam oxigenoterapia domiciliar permitiu que os autores identificassem 43 fumantes (17% da amostra como um todo) que até então haviam declarado não ser fumantes.(24) Por outro lado, um estudo realizado no Japão mostrou que, de 351 pacientes com DPOC ou asma, apenas 11 (2 com asma e 9 com DPOC) alegaram ser não fumantes e apresentaram concentração de cotinina sérica > 50 ng/ml, que sugere a presença de tabagismo ativo.(25) Esses resultados sugerem que diferenças culturais desempenham um papel na proporção de pacientes que tentam esconder seus hábitos de fumar de profissionais de saúde.
A inclusão de grupos de controle positivo e negativo em nosso estudo foi de grande importância para a discussão de pontos de corte na população estudada. Em nosso grupo controle positivo (de fumantes), a menor concentração de cotinina urinária foi de 458 ng/ml, e nenhum dos indivíduos apresentou concentração de COex < 10 ppm. Por outro lado, a maioria dos indivíduos do grupo controle negativo (indivíduos que nunca fumaram) apresentou concentração de cotinina < 100 ng/ml, e nenhum apresentou concentração de COex > 6 ppm. Resultados falso positivos de COex foram obtidos em 6 pacientes com DPOC e em apenas 1 paciente com asma. Todos os resultados positivos falsos ficaram dentro da faixa de 7-10 ppm, que é normalmente observada em fumantes leves. Todos os pacientes com resultados positivos falsos compartilhavam o domicílio com um fumante. Portanto, esses resultados poderiam ser explicados por exposição ambiental à fumaça de tabaco. A exposição à poluição e as doenças pulmonares inflamatórias subjacentes são também possíveis razões para os resultados positivos falsos dos testes de COex.
Em uma pesquisa anterior realizada em nossa instituição, concentrações de COex ≥ 6 ppm apresentaram a maior sensibilidade e especificidade para a diferenciação entre fumantes e não fumantes.(13) No entanto, quando se tenta identificar relatos falsos de tabagismo em pacientes com doenças pulmonares obstrutivas, essas diferenças claras desaparecem, e há um limite no qual ocorre sobreposição. Se usássemos os mais altos pontos de corte de COex sugeridos na literatura (11 ppm para a DPOC e 10 ppm para a asma), 32% de nossos pacientes com DPOC e 40% de nossos pacientes com asma seriam falsamente classificados em não fumantes, apesar de apresentarem concentrações de cotinina urinária > 200 ng/ml. No entanto, uma elevada proporção de nossos pacientes com concentrações de cotinina urinária < 100 ng/ml apresentaram níveis de COex entre 6 ppm e 8 ppm, o que ressalta a dificuldade em estabelecer um ponto de corte apropriado para o COex. Isso sugere que o nível de corte deve variar de população para população, e que resultados limítrofes devem ser avaliados com cuidado na prática clínica, especialmente porque é provável que o nível de exposição ambiental à fumaça do tabaco seja alto em pacientes com asma.(26)
A determinação precisa do tabagismo é fundamental para o tratamento da asma e da DPOC. Um estudo de "vida real" sobre a eficácia da terapia de cessação do tabagismo no Brasil mostrou que não houve associação entre comorbidades respiratórias e falha do tratamento. (27) Aconselhamento e tratamento farmacológico podem mudar o hábito de fumar dos pacientes e melhorar o curso da doença pulmonar.
O presente estudo tem algumas limitações. Não averiguamos a história de tabagismo passivo fora de casa, o último cigarro fumado ou os padrões de tabagismo. Esses fatores podem influenciar os níveis de COex e poderiam diminuir a sensibilidade e especificidade do monitoramento do COex. Além disso, não foram coletados dados demográficos relativos a características étnicas ou raciais. As diferenças étnicas ou raciais no metabolismo e depuração da nicotina poderiam constituir uma explicação alternativa para nossos achados. Há relatos de que a raça pode influenciar as concentrações de cotinina; os níveis de cotinina sérica são maiores em fumantes negros do que em fumantes brancos, em virtude de diferenças no metabolismo da nicotina.(28) Se a população brasileira metaboliza a nicotina de maneira mais lenta, nossos resultados seriam parcialmente explicados sem subnotificação. Outra explicação é que a obstrução das vias aéreas pode influenciar os níveis de COex. Portanto, as medidas de COex podem ser imprecisas em pacientes com obstrução grave das vias aéreas.(29) No entanto, embora os pacientes de nossa amostra apresentassem obstrução das vias aéreas de moderada a grave, não houve correlação significativa entre COex e VEF1. Outra possível limitação de nosso estudo é que foi usada uma amostra de conveniência de participantes consecutivos, e não uma amostra aleatória de indivíduos.
Os resultados de nosso estudo confirmam que a história de tabagismo relatada pelo paciente não é confiável, pois o tabagismo referido pelos pacientes não se correlacionou com a concentração de cotinina urinária ou de COex. Nossos achados também indicam que o COex é suficiente para distinguir fumantes de não fumantes. Se nossos resultados puderem ser generalizados para outras populações e doenças, então há motivo para preocupação com o uso de questionários como as únicas fontes de dados sobre o tabagismo em estudos epidemiológicos e pesquisas envolvendo pacientes com doenças respiratórias.
Em suma, o presente estudo corrobora a ideia de que o tabagismo autodeclarado não é confiável na população de pacientes com doenças pulmonares obstrutivas, já que uma considerável proporção de nossa amostra de pacientes mentiu para seus médicos. A medição objetiva do tabagismo pode ser útil para melhorar o tratamento clínico e aconselhamento de pacientes com DPOC e de pacientes com asma.
Referências1. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-65. http://dx.doi.org/10.1164/rccm.201204-0596PP
2. Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. Eur Respir J. 2004;24(5):822-33. http://dx.doi.org/10.1183/09031936.04.00039004
3. Price DB, Tinkelman DG, Halbert RJ, Nordyke RJ, Isonaka S, Nonikov D, et al. Symptom-based questionnaire for identifying COPD in smokers. Respiration. 2006;73(3):285-95. http://dx.doi.org/10.1159/000090142
4. Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ, Isonaka S, Nonikov D, et al. Symptom-based questionnaire for differentiating COPD and asthma. Respiration. 2006;73(3):296-305. http://dx.doi.org/10.1159/000090141
5. Corbo GM. Chronic obstructive pulmonary disease and asthma in the primary care setting: simple questionnaires can promote patient awareness and provide feedback to physicians. Respiration. 2006;73(3):277-8. http://dx.doi.org/10.1159/000092081
6. Gallus S, Tramacere I, Boffetta P, Fernandez E, Rossi S, Zuccaro P, et al. Temporal changes of under-reporting of cigarette consumption in population-based studies. Tob Control. 2011;20(1):34-9. http://dx.doi.org/10.1136/tc.2009.034132
7. Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12-24. http://dx.doi.org/10.1093/ntr/ntn010
8. Malta DC, Moura EC, Silva SA, Oliveira PP, Silva VL. Prevalence of smoking among adults residing in the Federal District of Brasília and in the state capitals of Brazil, 2008. J Bras Pneumol. 2010;36(1):75-83. http://dx.doi.org/10.1590/S1806-37132010000100013
9. Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161(2 Pt 1):381-90. http://dx.doi.org/10.1164/ajrccm.161.2.9901044
10. Glasgow RE, Mullooly JP, Vogt TM, Stevens VJ, Lichtenstein E, Hollis JF, et al. Biochemical validation of smoking status: pros, cons, and data from four low-intensity intervention trials. Addict Behav. 1993;18(5):511-27. http://dx.doi.org/10.1016/0306-4603(93)90068-K
11. Cunnington AJ, Hormbrey P. Breath analysis to detect recent exposure to carbon monoxide. Postgrad Med J. 2002;78(918):233-7. http://dx.doi.org/10.1136/pmj.78.918.233
12. Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163(7):1693-722. http://dx.doi.org/10.1164/ajrccm.163.7.2009041
13. Santos UP, Gannam S, Abe JM, Esteves PB, Freitas Filho M, Wakassa TB, et al. Use of breath carbon monoxide as an indicator of smoking status [Article in Portuguese]. J Pneumol. 2001;27(5):231-6. http://dx.doi.org/10.1590/S0102-35862001000500001
14. Haufroid V, Lison D. Urinary cotinine as a tobacco-smoke exposure index: a minireview. Int Arch Occup Environ Health. 1998;71(3):162-8. http://dx.doi.org/10.1007/s004200050266
15. Tricker AR. Nicotine metabolism, human drug metabolism polymorphisms, and smoking behaviour. Toxicology. 2003;183(1-3):151-73. http://dx.doi.org/10.1016/S0300-483X(02)00513-9
16. Murray RP, Connett JE, Lauger GG, Voelker HT. Error in smoking measures: effects of intervention on relations of cotinine and carbon monoxide to self-reported smoking. The Lung Health Study Research Group. Am J Public Health. 1993;83(9):1251-7. http://dx.doi.org/10.2105/AJPH.83.9.1251
17. Global Initiative for Asthma - GINA. [homepage on the Internet]. Bethesda: Global Initiative for Asthma. [cited 2014 Dec 3]. Global Strategy for Asthma Management and Prevention. Available from: www.ginaasthma.org
18. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-38. http://dx.doi.org/10.1183/09031936.05.00034805
19. Nakajima M, Yamamoto T, Kuroiwa Y, Yokoi T. Improved highly sensitive method for determination of nicotine and cotinine in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2000;742(1):211-5. http://dx.doi.org/10.1016/S0378-4347(00)00149-3
20. Zielinska-Danch W, Wardas W, Sobczak A, Szołtysek-Bołdys I. Estimation of urinary cotinine cut-off points distinguishing non-smokers, passive and active smokers. Biomarkers. 2007;12(5):484-96. http://dx.doi.org/10.1080/13547500701421341
21. Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117(3):758-63. http://dx.doi.org/10.1378/chest.117.3.758
22. Deveci SE, Deveci F, Acik Y, Ozan AT. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir Med. 2004;98(6):551-6. http://dx.doi.org/10.1016/j.rmed.2003.11.018
23. Lores Obradors L, Monsó Molas E, Rosell Gratacós A, Badorrey I, Sampablo Lauro I. Do patients lie about smoking during follow-up in the respiratory medicine clinic? [Article in Spanish]. Arch Bronconeumol. 1999;35(5):219-22.
24. Cornette A, Petitdemange I, Briancon S, Burlet C, Polu JM. Evaluation of smoking in chronic severe respiratory insufficiency patients treated with long-term oxygen at home [Article in French]. Rev Mal Respir. 1996;13(4):405-11.
25. Sato S, Nishimura K, Koyama H, Tsukino M, Oga T, Hajiro T, et al. Optimal cutoff level of breath carbon monoxide for assessing smoking status in patients with asthma and COPD. Chest. 2003;124(5):1749-54. http://dx.doi.org/10.1378/chest.124.5.1749
26. Dias-Júnior SA, Pinto RC, Angelini L, Fernandes FL, Cukier A, Stelmach R. Prevalence of active and passive smoking in a population of patients with asthma. J Bras Pneumol. 2009;35(3):261-5. PMid:19390725
27. Prado GF, Lombardi EM, Bussacos MA, Arrabal-Fernandes FL, Terra-Filho M, Santos Ude P. A real-life study of the effectiveness of different pharmacological approaches to the treatment of smoking cessation: re-discussing the predictors of success. Clinics (Sao Paulo). 2011;66(1):65-71. http://dx.doi.org/10.1590/S1807-59322011000100012
28. Signorello LB, Cai Q, Tarone RE, McLaughlin JK, Blot WJ. Racial differences in serum cotinine levels of smokers. Dis Markers. 2009;27(5):187-92. http://dx.doi.org/10.1155/2009/104647
29. Togores B, Bosch M, Agusti AG. The measurement of exhaled carbon monoxide is influenced by airflow obstruction. Eur Respir J. 2000;15(1):177-80. http://dx.doi.org/10.1183/09031936.00.15117700
*Trabalho realizado na Disciplina de Pneumologia, Instituto do Coração - InCor - Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
Endereço para correspondência: Frederico Leon Arrabal Fernandes. Avenida Eneás de Carvalho Aguiar, 44, Pneumologia, CEP 05403-900, São Paulo, SP, Brasil.
Tel: 55 11 3069-5034. E-mail: frederico.fernandes@incor.usp.br
Apoio financeiro: Nenhum.
Recebido para publicação em 4/12/2014. Aprovado, após revisão, em 11/2/2015.
Sobre os autoresRafael Stelmach
Médico Assistente. Disciplina de Pneumologia, Instituto do Coração - InCor - Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
Frederico Leon Arrabal Fernandes
Médico Assistente. Disciplina de Pneumologia, Instituto do Coração - InCor - Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
Regina Maria Carvalho-Pinto
Médica Assistente. Disciplina de Pneumologia, Instituto do Coração - InCor - Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
Rodrigo Abensur Athanazio
Médico Assistente. Disciplina de Pneumologia, Instituto do Coração - InCor - Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
Samia Zahi Rached
Médica Assistente. Disciplina de Pneumologia, Instituto do Coração - InCor - Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
Gustavo Faibischew Prado
Médico Assistente. Disciplina de Pneumologia, Instituto do Coração - InCor - Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
Alberto Cukier
Diretor Técnico. Disciplina de Pneumologia, Instituto do Coração - InCor - Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.







