Paulo Francisco Guerreiro Cardoso, Rogério Pazetti, Henrique Takachi Moriya, Paulo Manuel Pêgo-Fernandes, Francine Maria de Almeida, Aristides Tadeu Correia, Karina Fechini, Fabio Biscegli Jatene
ABSTRACT
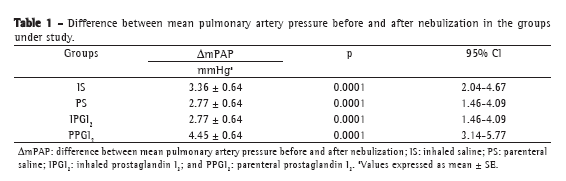
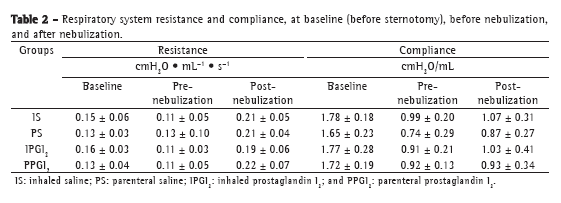
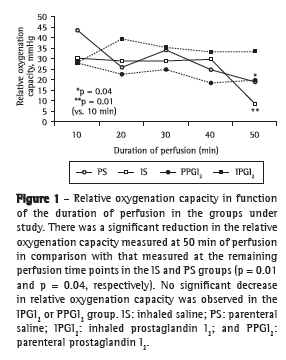
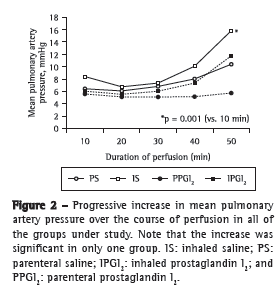
Objective: To present a model of prostaglandin I2 (PGI2) administration (inhaled vs. parenteral) and to assess the functional performance of the lungs in an ex vivo lung perfusion system. Methods: Forty Wistar rats were anesthetized and placed on mechanical ventilation followed by median sterno-laparotomy and anticoagulation. The main pulmonary artery was cannulated. All animals were maintained on mechanical ventilation and were randomized into four groups (10 rats/group): inhaled saline (IS); parenteral saline (PS); inhaled PGI2 (IPGI2); and parenteral PGI2 (PPGI2). The dose of PGI2 used in the IPGI2 and PPGI2 groups was 20 and 10 µg/kg, respectively. The heart-lung blocks were submitted to antegrade perfusion with a low potassium and dextran solution via the pulmonary artery, followed by en bloc extraction and storage at 4°C for 6 h. The heart-lung blocks were then ventilated and perfused in an ex vivo lung perfusion system for 50 min. Respiratory mechanics, hemodynamics, and gas exchange were assessed. Results: Mean pulmonary artery pressure following nebulization decreased in all groups (p < 0.001), with no significant differences among the groups. During the ex vivo perfusion, respiratory mechanics did not differ among the groups, although relative oxygenation capacity decreased significantly in the IS and PS groups (p = 0.04), whereas mean pulmonary artery pressure increased significantly in the IS group. Conclusions: The experimental model of inhaled PGI2 administration during lung extraction is feasible and reliable. During reperfusion, hemodynamics and gas exchange trended toward better performance with the use of PGI2 than that with the use of saline.
Keywords: Prostaglandins; Lung transplantation; Reperfusion; Models, animal; Rats.
RESUMO
Objetivo: Apresentar um modelo experimental de administração de prostaglandina I2 (PGI2) por via inalatória vs. parenteral e avaliar o desempenho funcional dos pulmões em um sistema de perfusão pulmonar ex vivo. Métodos: Quarenta ratos Wistar foram anestesiados, ventilados, submetidos a laparotomia com ressecção do esterno e anticoagulados. O tronco da artéria pulmonar foi canulado. Todos os animais foram submetidos a ventilação mecânica. Os animais foram randomizados em quatro grupos (10 ratos/grupo): salina nebulizada (SN); salina parenteral (SP); PGI2 nebulizada (PGI2N); e PGI2 parenteral (PGI2P). A dose de PGI2 nos grupos PGI2N e PGI2P foi de 20 e 10 µg/kg, respectivamente. Os blocos cardiopulmonares foram submetidos in situ a perfusão anterógrada com solução de baixo potássio e dextrana a 4°C via artéria pulmonar, extraídos em bloco e armazenados a 4°C por 6 h. Os blocos foram ventilados e perfundidos em um sistema ex vivo por 50 min, sendo obtidas medidas de mecânica ventilatória, hemodinâmica e trocas gasosas. Resultados: Houve redução da pressão arterial pulmonar média após a nebulização em todos os grupos (p < 0,001), sem diferença entre os grupos. Na perfusão ex vivo, a mecânica ventilatória não diferiu entre os grupos. Houve redução da capacidade relativa de oxigenação ao longo da perfusão nos grupos SN e SP (p = 0,04), e houve aumento significativo da pressão arterial pulmonar no grupo SN. Conclusões: O modelo experimental de administração de PGI2 na extração pulmonar é exequível e confiável. Na reperfusão, os resultados de hemodinâmica e de trocas gasosas demonstraram tendência a um melhor desempenho com o uso de PGI2 do que com solução salina.
Palavras-chave: Prostaglandinas; Transplante de pulmão; Reperfusão; Modelos animais; Ratos.
Introduction