ABSTRACT
The incidence of lung neoplasms is increasing in Brazil and in the world, probably as a result of the increase in smoking. Due to the greater number of cases, atypical presentations appear. We report the case of a 66-year-old hypertensive male smoker who presented progressive proximal muscular weakness and, in two months, evolved to dysphagia, dysphonia, and V-shaped skin lesions on the chest. A chest X-ray showed a spiculated pulmonary nodule in the right upper lobe. The biochemical analysis revealed elevated creatine kinase levels. After complementary tests and biopsies, the patient underwent right upper lobectomy. Histopathology showed a moderately differentiated adenocarcinoma. The overall analysis of the case and a review of the literature allow us to suggest that the clinical profile of the patient was a result of an overlap of two paraneoplastic syndromes (dermatomyositis and Lambert-Eaton myasthenic syndrome) secondary to lung adenocarcinoma.
Keywords:
Lung neoplasms; Paraneoplastic syndromes; Dermatomyositis; Lambert-Eaton myasthenic syndrome; Adenocarcinoma.
RESUMO
A incidência das neoplasias pulmonares vem aumentando no Brasil e no mundo, provavelmente como resultado do aumento do tabagismo.
Com o maior número de casos, surgem as apresentações atípicas. Relatamos o caso de um paciente do sexo masculino, 66 anos, tabagista
e hipertenso, que apresentava quadro de fraqueza muscular proximal progressiva e, em dois meses, evoluiu com disfagia para alimentos
sólidos, disfonia e lesões cutâneas em forma de "V" no tórax. O radiograma de tórax mostrou um nódulo pulmonar espiculado no lobo superior
direito. A análise bioquímica revelou aumento da creatinoquinase. Após exames complementares e biópsias, o paciente foi submetido
à lobectomia superior direita. A histopatologia evidenciou um adenocarcinoma moderadamente diferenciado. A análise global do caso e a
revisão de literatura permitem sugerir que o quadro clínico do paciente era resultante da sobreposição de duas síndromes paraneoplásicas, a
saber, a dermatomiosite e a síndrome miastênica de Lambert-Eaton, secundárias a um adenocarcinoma pulmonar.
Palavras-chave:
A incidência das neoplasias pulmonares vem aumentando no Brasil e no mundo, provavelmente como resultado do aumento do tabagismo.
IntroductionThe World Health Organization registered a total of 6 million cancer patients worldwide in the year 2000. According to those data, lung cancer was the most prevalent, accounting for approximately 1 million cases.(1) Due to the higher prevalence of the disease, previously unseen manifestations have appeared. We describe the clinical case of a patient with lung adenocarcinoma who had concomitant dermatomyositis and Lambert-Eaton myasthenic syndrome (LEMS).
Case reportA 66-year-old male patient presented with a two-month history of dysphagia for solid foods and progressive proximal muscular weakness, together with difficulty in climbing stairs and raising his arms. One week later, he presented dysphonia and worsening of the dysphagia, together with reddish, desquamative, and slightly pruriginous V-shaped lesions distributed on the chest and back. The patient reported uncharacteristic weight loss (10 kg) in the last three months. He had had poorly controlled hypertension for 25 years and was a smoker (50 pack-years). In addition, the patient presented left hemiparesis as a sequela of a cerebrovascular accident 19 years prior.
Physical examination confirmed the dysphonia and revealed dysarthria. Muscle strength was graded as follows: grade 3 proximal muscle strength (active motion against gravity without resistance) and grade 5 distal muscle strength (normal) in the right arm; grade 3 proximal muscle strength and grade 4+ distal muscle strength (active motion against gravity with some resistance) in the left arm; grade 3 proximal muscle strength and grade 5 distal muscle strength in the right leg; and grade 3 proximal muscle strength and grade 4+ distal muscle strength in the left leg.(2) The patient also presented the Babinski sign on the left side and slightly desquamative, exanthematous V-shaped lesions on the chest and back.
Laboratory tests presented the following results: creatine kinase levels of 898 U/L (ref. ≤ 174 U/L); aspartate aminotransferase levels of 76 U/L (ref. ≤ 35 U/L); antinuclear factor (ANF) in HEp-2 cells at 1:160 (fine dots); and nonreactive anti-SSA (Ro), anti-SSB (La), anti-Sm, and anti-RNP antibodies and rheumatoid factor. A chest X-ray showed a spiculated pulmonary nodule, with a diameter of 2 cm, in the left upper lung field. Computed tomography of the chest revealed a spiculated, dense nodule in the right pulmonary apex and presenting striations toward the pleura (Figure 1). The results of endoscopy of the upper digestive tract, bronchoscopy, and pulmonary function tests were normal. The results of the other relevant tests are presented in Chart 1.


The patient was given a presumed diagnosis of lung neoplasm with clinical manifestations of two overlapping paraneoplastic syndromes: dermatomyositis and LEMS.
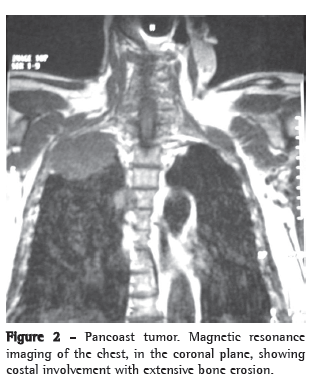
The patient underwent right upper lobectomy with mediastinal lymphadenectomy. Histopathology showed a moderately differentiated adenocarcinoma that was pathologically staged as T1 N2 Mx (Figure 2).
In the postoperative period, the patient presented partial and spontaneous improvement of the skin lesions and dysphagia. Fifteen days later, he developed nosocomial pneumonia and, subsequently, septic shock. The patient died sixteen days after the surgical procedure.
DiscussionIn Brazil, data obtained from the National Cancer Institute reveal that, in terms of incidence, lung cancer is the second leading type of cancer among men and the third among women, increasing among females due to the increase in the number of female smokers. In 2006, 27,170 Brazilians (17,850 men and 9320 women) were diagnosed with lung cancer.(3)
In 90% of all cases, lung cancer can be classified as one of four histological types: epidermoid carcinoma; adenocarcinoma; large cell carcinoma; or small cell carcinoma. From a practical, treatment standpoint, individuals with lung cancer are divided into two groups: those who have small cell lung cancer and those who have non-small cell lung cancer.(4,5)
Clinical manifestations can be related to the location of the primary tumor, the invasion of adjacent structures, metastases, and paraneoplastic manifestations.(4-7) Adenocarcinoma is the histological type most commonly found among nonsmokers and women, being characterized by its peripheral location and by the early appearance of metastases.(5) In addition, it can manifest as paraneoplastic syndromes, among which hypertrophic osteoarthropathy is the most common.(4)
The patient in question differed from the usual descriptions found in the literature by being a smoking male and having developed a lung adenocarcinoma with clinical manifestations of the superimposition of two uncommon paraneoplastic syndromes.
Dermatomyositis includes a heterogeneous group of inflammatory diseases that affect the skin and the skeletal muscles.(8) The classical clinical profile is defined as characteristic skin involvement and progressive proximal weakness, accompanied by an increase in muscle enzyme levels, as well as by electromyogram and muscle biopsy results that are suggestive of the disease.(9)
Skin manifestations include heliotrope rash (violaceous erythematous rash with symmetrical peri-orbital distribution) and Gottron's papules (violaceous papules or maculae distributed on the extensor surfaces of the proximal and distal metacarpophalangeal and interphalangeal joints). Other manifestations, such as mechanic's hand or V-shaped skin rash on the chest and back, can occur.(7,10,11) Skin biopsy can show chronic perivascular and peri-adnexal inflammation, and mucin deposits are common findings.(12) In addition, CD4+ cells can be found in the dermis.(11)
Muscle involvement is suspected when proximal and progressive symmetric weakness, manifesting as inability to climb stairs or raise the arms, is reported.(10,11,13) Muscle enzyme levels are usually high. The most specific marker is the creatine kinase level, which is related to the severity of the disease.(11) Over the course of the disease, dysphagia and dysphonia due to the involvement of the striated musculature of the pharynx and proximal esophagus is common, which means a worse prognosis due to the higher incidence of complication related to bronchial aspiration.(10,11) Muscle biopsy reveals inflammatory infiltrate near the muscle vessels and fibers, as well as fiber atrophy in the periphery of the fascicles.(11)
Testing for auto-antibodies is typically ANF-positive, although the result has no relation to diagnosis or prognosis.(11) The level of anti-Mi2 is specific for dermatomyositis, although it presents low sensitivity (positive in 25% of the cases).(8,11)
Although the association between dermatomyositis and neoplasia is known, it remains poorly understood.(13-16) Neoplasia can precede myositis, appear concomitantly, or be diagnosed after its clinical manifestation, with a higher frequency in the first three years after diagnosis.(7,13,15-17) In some cases, dermatomyositis follows the clinical course of the underlying neoplasm, both manifesting concomitantly and the former improving with the treatment of the latter, which suggests a paraneoplastic relationship between the two.(8,10,13,14,17)
Determining the presence of occult neoplasia must be part of the investigation of every patient diagnosed with dermatomyositis.(13,15) In a retrospective analysis of 618 patients diagnosed with dermatomyositis, 198 cases of cancer were reported (32% of the patients), and, in 83 (7%) of the patients, the two diseases were diagnosed concomitantly.(17) The investigation of a possible neoplasm should be guided by local epidemiology, risk factors, age (increased risk in individuals over 50), and gender.(10,13,15,16)
The relationship between lung cancer and connective tissue diseases has been described, and the incidences of rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, and dermatomyositis are variable.(15,16) In a study that analyzed 153 cases of lung cancer accompanied by connective tissue disease, 21 (13%) of the patients had dermatomyositis, and 3 (2%) had polymyositis. The epidemiological analysis of the group in question revealed a predominance of males (19/24; 79%), with a mean age of 57 years. Regarding the histological type, there was a higher prevalence of small cell carcinoma (7/24; 29%) and epidermoid carcinoma (5/24; 20%). Only 8.3% (2/24) of the cases reported were adenocarcinoma.(15,16) In most patients, dermatomyositis or polymyositis was diagnosed slightly prior to or concomitantly with the lung cancer.(16)
A characteristic finding in LEMS is a defect in the release of acetylcholine in the autonomous nervous system and in the presynaptic terminals of the neuromuscular junction.(7,18) Proximal muscle fatigability of insidious onset is the most common manifestation.(7,18) Atrophy, hyporeflexia, and temporary involvement of cranial pairs, manifesting as diplopia, ptosis, or dysphagia, can be found.(18)
The diagnosis should be confirmed through electromyography, which reveals alterations that are characteristic of LEMS.(7,18) In cases of paraneoplastic LEMS, tumor resection, combined with chemotherapy or radiotherapy, results, in great part, in normalization of the electromyography findings and regression of the clinical manifestations.(7,18,19)
Concomitant small cell lung cancer is seen in 50 to 70% of LEMS cases, whereas other malignancies, such as non-small cell lung cancer, uterine cervical cancer, prostate cancer, and lymphoma, are less common.(7,19)
The skin lesions presented by the patient, the esophageal manometry findings revealing upper esophageal sphincter hypotonia, the muscle biopsy findings showing alterations consistent with myopathy accompanied by the increase in muscle enzyme levels, and the ANF positivity confirmed the diagnosis of dermatomyositis. Similarly, the electromyography findings were characteristic of LEMS.
In view of these facts, it is possible to correlate the clinical profile of asthenia, proximal muscle weakness, and dysphagia presented by the patient as being a result of the overlap of the two syndromes reported, both being paraneoplastic in nature and secondary to lung adenocarcinoma.
References 1. GLOBOCAN 2000. Cancer Incidence, Mortality and Prevalence Worldwide. Version 1.0. Lyon: IARCPress; 2001.
2. Miller DW, Hahn JF. General methods of clinical examination. In: Youmans JR, editor. Neurological Surgery: A Comprehensive Reference Guide to the Diagnosis and Management of Neurosurgical Problems. 4th ed. Philadelphia: W. B. Saunders; 1996. p. 31-32.
3. Estimativas para o ano de 2006 de números de casos novos por câncer, em homens e mulheres, segundo localização primária (Brasil). In: Estimativa 2006: Incidência de câncer no Brasil. Rio de Janeiro: INCA; 2005. p. 39-40.
4. Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician. 2007;75(1):56-63.
5. Hoffman PC, Mauer AM, Vokes EE. Lung cancer.Lancet. 2000;355(9202):479-85.
6. Koler RA, Montemarano A. Dermatomyositis. Am Fam Physician. 2001;64(9):1565-72.
7. Lin JT, Lachmann E. Lambert-eaton myasthenic syndrome: a case report and review of the literature. J Womens Health (Larchmt). 2002;11(10):849-55.
8. Dourmishev LA, Dourmishev AL, Schwartz RA. Dermatomyositis: cutaneous manifestations of its variants. Int J Dermatol. 2002;41(10):625-30.
9. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292(7):344-7.
10. Callen JP. Dermatomyositis. Lancet. 2000;355(9197):53-7.
11. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971-82.
12. del Pozo J, Almagro M, Martínez W, Yebra-Pimentel MT, García-Silva J, Peña-Penabad C, et al. Dermatomyositis and mucinosis. Int J Dermatol. 2001;40(2):120-4.
13. Fam AG. Paraneoplastic rheumatic syndromes. Baillieres Best Pract Res Clin Rheumatol. 2000;14(3):515-33.
14. Stone SP, Buescher LS. Life-threatening paraneoplastic cutaneous syndromes. Clin Dermatol. 2005;23(3):301-6.
15. Yang Y, Fujita J, Tokuda M, Bandoh S, Ishida T. Lung cancer associated with several connective tissue diseases: with a review of literature. Rheumatol Int. 2001;21(3):106-11.
16. Fujita J, Tokuda M, Bandoh S, Yang Y, Fukunaga Y, Hojo S, et al. Primary lung cancer associated with polymyositis/dermatomyositis, with a review of the literature. Rheumatol Int. 2001;20(2):81-4.
17. Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96-100.
18. Stojic AS, Mekhail T, Tsao BE. Leg weakness in a 66-year-old woman: a common presentation of an uncommon disease. Cleve Clin J Med. 2007;74(1):23-6, 29-34.
19. de Beukelaar JW, Sillevis Smitt PA. Managing paraneoplastic neurological disorders. Oncologist. 2006;11(3):292-305.
____________________________________________________________________________________________________________________
Study carried out in the Department of Respiratory Diseases of the Hospital do Servidor Público Estadual Francisco Morato de Oliveira - HSPE/FMO, Francisco Morato de Oliveira Hospital for State Civil Servants - São Paulo, Brazil.
1. Resident Physician. Hospital do Servidor Público Estadual Francisco Morato de Oliveira - HSPE/FMO, Francisco Morato de Oliveira Hospital for State Civil Servants - São Paulo, Brazil.
2. Physician in the Department of Respiratory Diseases. Hospital do Servidor Público Estadual Francisco Morato de Oliveira - HSPE/FMO, Francisco Morato de Oliveira Hospital for State Civil Servants - São Paulo, Brazil.
3. Physician in the Department of Pathology. Hospital do Servidor Público Estadual Francisco Morato de Oliveira - HSPE/FMO, Francisco Morato de Oliveira Hospital for State Civil Servants - São Paulo, Brazil.
Correspondence to: Fernanda Manente Milanez. Hospital do Servidor Público Estadual. Departamento de Doenças do Aparelho Respiratório (10º andar). Av. Ibirapuera, 981, Bairro Vila Clementino, CEP 04029-000, São Paulo, SP, Brasil.
Tel 55 11 5088-8292. E-mail: femanente@terra.com.br
Submitted: 15 May 2007. Accepted, after review: 31 July 2007.




