ABSTRACT
Objective: The translation and cross-cultural adaptation of a specific scoring instrument for the comprehensive control of asthma, the Asthma Control Scoring System (ACSS), for use in Brazil. Methods: The protocol included ten steps: acquisition of written permission from the author of the ACSS; translation of the instrument to Brazilian Portuguese, carried out by three separate translators; analysis and comparison of the three versions by a review committee; literal back-translation to English; review and harmonization of the back-translation; acquisition of the approval of the original author; review of the translation by specialists; cognitive debriefing: test of clarity to, understanding by, and acceptance of the target population (evaluation of the translation by 10 health care workers); second cognitive debriefing: review of the revised version by a second group of health care workers; and reconciliation and preparation of the final version by the review committee. Results: The Brazilian Portuguese-language version of the ACSS showed clarity, understandability, and acceptability. The instrument was considered to be comprehensive because it includes the clinical manifestations of asthma, as well as the functional and inflammatory aspects of the disease. Conclusions: With the use of this careful methodology in the translation and cross-cultural adaptation of the ACSS, we have ensured its cultural adequacy for Brazil. The use of this instrument could facilitate future studies on asthma control.
Keywords:
Asthma/classification; Asthma/prevention & control; Questionnaires.
RESUMO
Objetivo: Traduzir e adaptar culturalmente, para uso no Brasil, um instrumento específico de escore para o controle abrangente da asma, denominado Asthma Control Scoring System (ACSS). Métodos: O protocolo incluiu dez etapas: autorização escrita do autor do ACSS; tradução do instrumento para a língua portuguesa do Brasil por três tradutores; análise e comparação das três versões por um comitê revisor; retradução literal para o inglês; revisão e harmonização da retradução; aprovação do autor do ACSS; revisão da tradução por especialistas; desdobramento cognitivo: teste da clareza, compreensão e aceitabilidade junto à população alvo (avaliação da tradução por 10 profissionais da área da saúde); segundo desdobramento cognitivo: revisão da nova versão por um segundo grupo de profissionais da área de saúde; e reconciliação e elaboração da versão final pelo comitê revisor. Resultados: A versão do ACSS em português do Brasil apresentou clareza, compreensão e aceitabilidade. O instrumento foi considerado abrangente por englobar as manifestações clínicas, funcionais e inflamatórias da asma. Conclusões: Com o uso desta metodologia criteriosa empregada para a adaptação transcultural do ACSS, asseguramos sua adequação cultural para uso no Brasil. O uso desse instrumento poderá facilitar futuros estudos sobre o controle da asma.
Palavras-chave:
Asma/classificação; Asma/prevenção & controle; Questionários.
IntroductionCurrent guidelines indicate that the primary goal of asthma management is to achieve and maintain control of the disease in order to reduce unfavorable outcomes, such as exacerbations and loss of respiratory function over time.(1-4) Uncontrolled asthma, in addition to affecting the quality of life of patients,(5,6) increases disease-related costs, which are due to a greater number of emergency room visits and hospitalizations, as well as the indirect costs related to absenteeism.(7,8)
Asthma control can be defined in many ways and might have a different meaning to physicians than it has to patients. In general, the term "asthma control" is applied to the control of the clinical manifestations and functional aspects of the disease, although it should also include the control of inflammation.(1) Recent evidence(9) suggests that the control of eosinophilic inflammation of the airways, monitored by sputum cell counts, is associated with a significant reduction in the number of exacerbations.
However, the use of objective measures to monitor airway inflammation in asthma is not yet routine.(10,11) Consequently, national and international guidelines(1,2) recommend that treatment should be directed at controlling only the clinical and functional manifestations of the disease.
According to the Global Initiative for Asthma (GINA), asthma should be classified as controlled, partially controlled, and uncontrolled based on the assessment of symptoms, physical activity, and pulmonary function.(1)
Therefore, a patient with controlled asthma should be free of daytime symptoms, nocturnal awakenings, the need for rescue medication, and activity limitations, as well as having normal or near-normal pulmonary function and having had no exacerbations in the past year.
In addition to the criteria proposed by the guidelines, asthma control can be measured by instruments specifically designed for that purpose. Currently, there are two asthma control questionnaires that have been cross-culturally adapted, translated, and validated for use in Brazil: the Asthma Control Questionnaire (ACQ)(12,13); and the Asthma Control Test (ACT).(14) Recently, Boulet et al.(15) developed and validated another questionnaire, the Asthma Control Scoring System (ACSS), which differs from the others by including airway inflammation as an additional determinant of asthma control. The ACSS includes three domains: clinical; physiological; and airway inflammation. The clinical domain addresses symptoms, use of β2 agonists as rescue medication, and activities performed in the past week.
The physiological domain refers to the measurement of FEV1 or PEF. The airway inflammation domain results from the analysis of the proportion of eosinophils in induced sputum. The ACSS has a total of eight items, which are scored in percentage terms, and the maximum total score is 100%. A score of 100% indicates totally controlled asthma; a score between 80% and 99% indicates adequately controlled asthma; a score between 60% and 79% indicates poorly controlled asthma; a score between 40% and 59% indicates very poorly controlled asthma; and a score below 40% indicates uncontrolled asthma.(15)
For the ACSS to be used in Brazil, it should be translated and adapted to the social and cultural circumstances of the country. Therefore, the objective of the present study was the translation and cross-cultural adaptation of the ACSS, by means of a rigorous methodology, for use in Brazil.
MethodsThis study involved the cross-cultural adaptation, as well as the translation to Brazilian Portuguese, of a specific scoring instrument for the comprehensive control of asthma-the ACSS. This instrument consists of an "ACSS User's Guide", "Instructions for the use of the ACSS", and a "Scoring grid". The present study was approved by the Human Research Ethics Committee of the Federal University of Santa Catarina and was conducted in accordance with established ethical principles.
In the methodology of the process of cross-cultural adaptation of the ACSS, the steps of the process of cross-cultural adaptation and validation were carried out in accordance with current guidelines.(16,17) The steps involved were as follows:
1) Preparation: The first author of the study contacted the author of the ACSS, obtaining permission and the rights of use, translate, and cross-culturally adapt the instrument.
2) Translation of the ACSS from English to Brazilian Portuguese: Three native speakers of Portuguese, who were fluent in English, aware of the objective of the study, and blinded to the work of the other translators, independently translated the ACSS. In this phase, emphasis was placed on a conceptual translation rather than on a literal translation.
3) Reconciliation: Those three versions were analyzed and compared, item by item, with the original English version of the instrument. Discrepancies were documented and analyzed by a review committee consisting of three pulmonologists and a physiotherapist specializing in respiratory therapy, and a single Brazilian Portuguese-language version (designated Brazilian Portuguese-language version 1) was prepared.
4) Back-translation: In this phase, version 1 was literally translated back to English. A fourth translator, who was an English teacher, born in an English-speaking country but now fluent in Portuguese, was selected for the task. This translator had no access to the original English version of the ACSS, and the translation was as literal as possible.
5) Review and harmonization of the back-translation: The review committee compared the back-translation with the original English version. Possible misunderstandings and translation errors due to difficulties in understanding the instrument were identified. The differences observed between the Brazilian Portuguese-language version and the English version were highlighted, and, subsequently, a harmonized Brazilian Portuguese-language version (designated Brazilian Portuguese-language version 2) was created.
6) Acquisition of the approval of the author of the ACSS: The second English-language version was sent to the author of the ACSS for evaluation and comments on consistency. After the back-translation had been approved by the author of the original version, another Brazilian Portuguese-language version (version 3), incorporating the corrections and suggestions made by the author of the ACSS, was prepared.
7) Review of version 3 of the ACSS by specialists: Two pulmonologists were invited to comment on and evaluate the adapted instrument. These comments were discussed with the author of the ACSS and analyzed jointly before another Brazilian Portuguese-language version of the instrument (Brazilian Portuguese-language version 4) was created. Version 4 was evaluated by a professor of Portuguese, who revised the text.
8) First cognitive debriefing: The objective of this step was to assess clarity of wording and to identify problematic issues in the instrument as a whole, therefore providing solutions to facilitate understanding of the instrument. Because the ACSS is an instrument used by the interviewer (a health care worker), five pulmonologists and five physiotherapists specializing in respiratory therapy were invited to participate in this phase of the study. The study was fully explained to the participants, and the instrument was offered, by the same researcher, to each of them. The participants were asked about their understanding and acceptability of each statement. The clarity of each statement, that is, the understanding of the wording of each statement by the participants, was graded from 1 to 10. Scores between 1 and 4 were defined as indicating a confusing statement, which should be replaced; scores between 5 and 7 indicated an unclear statement, which should be corrected; and scores between 8 and 10 indicated a clear statement.(18) In order to assess the clarity, acceptability, and understandability of the instrument, each participant was asked to register a comment on each item, sentence, or assertion. A clarity index was calculated as the mean sum of the grades given by the interviewees.(18) The statements (items) that did not obtain a clarity index above 0.8 were reworded by the review committee, who replaced certain terms with others that expressed the same concept so that the structure and assessment properties of the instrument were not significantly changed. Some instruction formats were changed, and inappropriate sentences were restructured to avoid misinterpretation in terms of consistency. Therefore, with the analysis of the comments made by the participants, another Brazilian Portuguese-language version (Brazilian Portuguese-language version 5), including the necessary corrections and adjustments, was prepared.
9) Second cognitive debriefing: Version 5 of the ACSS was submitted to a second group consisting of five pulmonologists and five physiotherapists specializing in respiratory therapy, all of whom were invited in the same way as the previous target population, with the objective of determining whether any statement was still considered inappropriate and whether the clarity index was above 0.8.(18)
10) Reconciliation and preparation of the final version: The objective of this step was to create the final version of the adapted instrument for use in Brazil. All of the participants in the previous steps, except for the participants in the cognitive debriefing step, met to create the final version of the process of translation and cross-cultural adaptation of the ACSS (translated as Sistema de Escore para Controle Abrangente da Asma-Scoring System for the Comprehensive Control of Asthma) for use in Brazil. As an illustration, all of the steps of the cross-cultural adaptation are shown in Figure 1.
 ResultsSteps of reconciliation, back-translation, review, and harmonization of the ACSS
ResultsSteps of reconciliation, back-translation, review, and harmonization of the ACSSIn the step of reconciliation of the ACSS, the review committee discussed and standardized the terms that were part of Brazilian Portuguese-language version 1. The back-translation of version 1 was accepted almost in its entirety by the review committee, and only one small correction was made to a sentence of the original "ACSS User's Guide": a "(d)" was added to "[...] excluding 1 dose per day before exercise", which therefore read "[...] excluding 1 dose (d) per day before exercise".
Acquisition of the approval of the original authorThe author of the ACSS approved the back-translation without any questions or corrections. He also reported that, in reviewing the instrument, he observed some deficiencies in clarity that could be overcome in the final Brazilian Portuguese-language version.
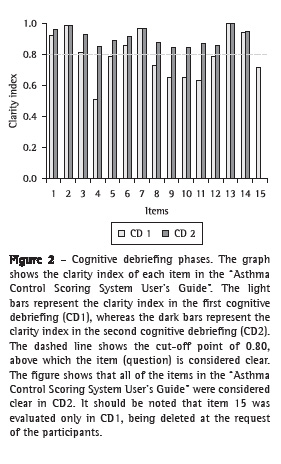
First cognitive debriefingIn this phase, some items of the "ACSS User's Guide" caused comprehension difficulties (Figure 2). Regarding the instructions of the "clinical component", item 4 (nocturnal symptoms) created the greatest difficulty, being considered clear by only 51% of the participants. The low rate of clarity was due to the length of the statement. The interviewees requested that it be divided into paragraphs and that one term be changed.
The item "physical activity" also obtained a low clarity index (.079, below the stipulated lower limit), because the interviewees reported a possible confusion between the terms "physical activity" and "physical exercise".
The instructions of the "inflammatory component" raised no questions, nor were there requests for changes, and the clarity index was 1.0. Eighty percent of the participants requested the inclusion of the meanings of the percentage ranges of the "Total score" in this guide. Despite the fact that item 15 was considered relevant, its deletion was requested because this item was not included in the "Scoring grid". We explained that, actually, item 15 was not part of the original published version, but that, in practice, it was used by the author's team. Item 15 addresses (school/work) absenteeism and the number of courses of prednisone used in the past year. The author of the ACSS, after having been contacted, agreed that these data, although interesting, did not appear in the original version of the ACSS and had no impact on the assessment of asthma control, because these data were not analyzed in the scoring grid of the ACSS.
Therefore, with the consent of the author, we removed item 15 from our final version. Chart 1 presents the modified items in the "ACSS User's Guide".
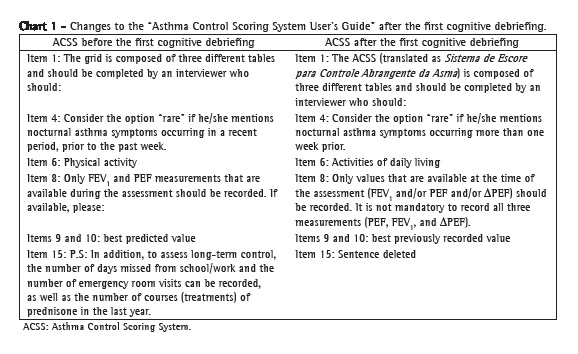
The "Instructions for the use of the ACSS", which contain the "Scoring grid", were well accepted and caused few misunderstandings. Most of the interviewees requested that each table be titled after the components (clinical, physiological, and inflammatory). Chart 2 presents the modified items.
 Second cognitive debriefing
Second cognitive debriefingIn this step, the clarity index of the "ACSS User's Guide", as well as that of the "Instructions for the use of the ACSS", was above 0.8 for all of the items (Figures 2 and 3).
 Reconciliation and preparation of the final version
Reconciliation and preparation of the final versionThe resulting final version (Appendix 1, supplementary online material-in Portuguese only) incorporated all of the changes described above. In this final version, the instructions are presented in a single document, the "Instruções para a Utilização do Sistema de Escore para Controle Abrangente da Asma (ACSS - Asthma Control Scoring System)" ("Instructions for the use of the ACSS"), with step-by-step instructions, according to the order in which the scoring grid is to be completed, facilitating the reading and making it easier to distinguish the fields for completion. In addition, the identification of each table (clinical component, physiological component, and inflammatory component) was added to the scoring grid. Finally, the final rating score (Total score), as well as the meaning of each percentage range, was added.
DiscussionIn this study, we used a careful methodology(16,17) in the translation and cross-cultural adaptation of the asthma control questionnaire. The choice for the cross-cultural adaptation of this instrument, rather than for the development of a new instrument, was based on three major factors. First, validated questionnaires are precision instruments.(19) Second, the translation and cross-cultural adaptation of a validated instrument makes it possible to compare results across studies conducted in different countries.(20) Finally, the construction of a questionnaire is an arduous and expensive task, and the validation of its measurement properties requires a considerable amount of time.(16)
The translation and cross-cultural adaptation of a validated questionnaire is a complex process that involves much more than simple translation,(21) because each item should be cross-culturally adapted in order to retain the conceptual meaning of the original questionnaire.(20) The methodology that we used in the process of translation and cross-cultural adaptation of the ACSS ensured the technical and semantic equivalence between the source version and the version created for use in Brazil. By ensuring such equivalence, we expect to maintain the psychometric properties of the ACSS, which have been reported in previous studies.(15,16,21) This has direct implications for the future applicability of the ACSS in Brazil, providing an instrument to measure the comprehensive control of asthma that retains the sensitivity of the original questionnaire.
Despite being technically and semantically equivalent to the original instrument, the Brazilian Portuguese-language version of the ACSS corrected and clarified minor discrepancies in the instructions to the original document. For example, in the original version of the ACSS, the instructions for its use are presented in the "ACSS User's Guide" and also in the "Instructions for the use of the Asthma Control Scoring System", resulting in repetition of information and lack of clarity due to the fact that the terms are not standardized. In addition, item 15, which addresses absenteeism, was deleted, because, although the author of the original version agreed that this information is interesting, the item did not appear in the original version of the ACSS and had no impact on the determination of the asthma control score. Because of these small changes, despite the fact they are perceived as unnecessary if a careful method of cross-cultural adaptation has been used, we consider that further studies employing the Brazilian Portuguese-language version of the ACSS are required in order to confirm whether this version of the ACSS provides equally appropriate and valid measurements.
Recently, various instruments to assess asthma control have attracted the interest of health care workers. Of those, two self-report questionnaires, the ACQ(12,13) and the ACT,(14) have been translated and validated for use in Brazil. At first, the reader could ask why adapt one more asthma control questionnaire. The choice for the cross-cultural adaptation of the ACSS for use in Brazil was based on the fact that it has a number of distinctions that can be seen as advantages. These advantages include the following: completion by health care workers; a score expressed as a percentage, which can facilitate its interpretation; the possibility of using the best previous FEV1 or PEF value recorded for patients with bronchial remodeling or small airway disease; and the measurement of airway inflammation by induced sputum.(15) Finally, the ACSS also allows clinical, physiological, and inflammatory parameters to be assessed in combination or separately.
How can the ACSS contribute to clinical practice? The basis for the use of the ACSS is the observation that it assesses different domains that comprise what we understand as asthma control. Many asthma patients consider their asthma to be well controlled, despite frequent symptoms, requiring physicians to ask specific questions for each of the various manifestations of the disease. These patients do not recognize or perceive the severity of the symptoms, which increases their risk for exacerbations.(22) Therefore, monitoring the level of control by assessing different domains is an integral and essential part of the management of asthma patients.(1,23) Some authors have stated that, in a superficial assessment, the parameters used in clinical practice can erroneously classify a patient with poorly controlled asthma as having well controlled asthma,(24) and this can consequently result in insufficient treatment and a higher risk of morbidity. In addition, overestimating severity can lead to an excessive use of drugs, unnecessarily increasing costs(25) and risks, with potential adverse effects of treatment.
The most important distinction of the ACSS is the measurement of eosinophilic inflammation of the airways. The inflammatory process is considered central to the pathogenesis of asthma, especially to the occurrence of exacerbations.(26) Recent studies have shown that, although symptom analysis alone is a sensitive indicator of changes in airflow or airway responsiveness, it can often have low sensitivity or be nonspecific for identifying an inflammatory process.(27) Some studies have demonstrated that asthma management based on induced sputum cell counts results in adequate control of current asthma-related limitations and a reduction in future risk, especially with regard to the number of exacerbations and the quantity of drugs needed in order to obtain control, thereby reducing the occurrence of adverse effects.(9,28) Unfortunately, the sputum induction method has yet to be widely used in clinical practice. However, there have been efforts to popularize the analysis of airway inflammation as an asthma control parameter, and such analysis has recently been listed in the GINA guidelines as a method that benefits patients with difficult-to-control asthma.(1) Therefore, the ACSS could be useful for physicians, in their clinical practice, improving the assessment and control of asthma in all of its components.(15)
In the present study, the process of translation and cross-cultural adaptation was carried out in accordance with current guidelines, and we are certain that the resulting Brazilian Portuguese-language version of the ACSS is a reliable tool for the assessment of asthma control. In addition, in order to obtain a better understanding of asthma control, we suggest that future studies comparing the various instruments that purport to assess the level of asthma control be carried out, thereby making it possible to construct a broader definition of asthma control.
AcknowledgmentsWe would like to thank the health care workers who participated in the cognitive debriefings. Our special thanks go to Dr. Louis-Philippe Boulet for authorizing the use of the ACSS and for his aid in the process of cross-cultural adaptation of the instrument.
References 1. Global Initiative for Asthma - GINA [homepage on the Internet]. Bethesda: Global Initiative for Asthma. [cited 2010 Mar 26]. Global Strategy for Asthma Management and Prevention, 2009. Available from: http://www.ginasthma.org
2. Sociedade Brasileira de Pneumologia e Tisiologia. IV Diretrizes Brasileiras para o Manejo da Asma 2006. J Bras Pneumol. 2006;32(Suppl 7);S447-S474.
3. Boulet LP, Becker A, Bérubé D, Beveridge R, Ernst P. Canadian Asthma Consensus Report, 1999. Canadian Asthma Consensus Group. CMAJ. 1999;161(11 Suppl):S1-61.
4. Clancy K. British guidelines on the management of asthma. Thorax. 2004;59(1):81-2.
5. Juniper EF, Wisniewski ME, Cox FM, Emmett AH, Nielsen KE, O'Byrne PM. Relationship between quality of life and clinical status in asthma: a factor analysis. Eur Respir J. 2004;23(2):287-91.
6. Andersson F, Borg S, Ståhl E. The impact of exacerbations on the asthmatic patient's preference scores. J Asthma. 2003;40(6):615-23.
7. Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996;9(4):636-42.
8. Santos LA, Oliveira MA, Faresin SM, Santoro IL, Fernandes AL. Direct costs of asthma in Brazil: a comparison between controlled and uncontrolled asthmatic patients. Braz J Med Biol Res. 2007;40(7):943-8.
9. Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemière C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27(3):483-94.
10. Voshaar T, App EM, Berdel D, Buhl R, Fischer J, Gessler T, et al. Recommendations for the choice of inhalatory systems for drug prescription [Article in German]. Pneumologie. 2001;55(12):579-86.
11. Gibson PG, Powell H. Written action plans for asthma: an evidence-based review of the key components. Thorax. 2004;59(2):94-9.
12. Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902-7.
13. Leite M, Ponte EV, Petroni J, D'Oliveira Júnior A, Pizzichini E, Cruz AA. Evaluation of the asthma control questionnaire validated for use in Brazil. J Bras Pneumol. 2008;34(10):756-63.
14. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59-65.
15. LeBlanc A, Robichaud P, Lacasse Y, Boulet LP. Quantification of asthma control: validation of the Asthma Control Scoring System. Allergy. 2007;62(2):120-5.
16. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46(12):1417-32.
17. Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8(2):94-104.
18. Melo SI. Coeficiente de atrito: um sistema de avaliação [thesis]. Santa Maria: Universidade Federal de Santa Maria; 1994.
19. Juniper EF. Validated questionnaires should not be modified. Eur Respir J. 2009;34(5):1015-7.
20. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-91.
21. Juniper EF. Medical questionnaires are copyrighted to ensure that validity is maintained. Chest. 2009;136(4):951-2.
22. Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O'Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. Am J Respir Crit Care Med. 1999;160(2):594-9.
23. Bousquet J, Clark TJ, Hurd S, Khaltaev N, Lenfant C, O'byrne P, et al. GINA guidelines on asthma and beyond. Allergy. 2007;62(2):102-12.
24. Bateman ED, Bousquet J, Braunstein GL. Is overall asthma control being achieved? A hypothesis-generating study. Eur Respir J. 2001;17(4):589-95.
25. Accordini S, Bugiani M, Arossa W, Gerzeli S, Marinoni A, Olivieri M, et al. Poor control increases the economic cost of asthma. A multicentre population-based study. Int Arch Allergy Immunol. 2006;141(2):189-98.
26. Tattersfield AE, Knox AJ, Britton JR, Hall IP. Asthma. Lancet. 2002;360(9342):1313-22.
27. Pizzichini E. Defining asthma control: time to look for new definitions?. J Bras Pneumol. 2007;33(6):xxxiv-vi.
28. Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715-21.
* Study carried out at the Núcleo de Pesquisa em Asma e Inflamação das Vias Aéreas - NUPAIVA, Center for Research on Asthma and Airway Inflammation - Polydoro Ernani de São Thiago University Hospital, Universidade Federal de Santa Catarina - UFSC, Federal University of Santa Catarina - Florianópolis, Brazil.
Correspondence to: Emílio Pizzichini. NUPAIVA, Hospital Universitário da UFSC, Campus Universitário, Trindade, CEP 88040-970, Florianópolis, SC, Brasil.
Tel/Fax: 55 48 3234-7711. E-mail: pizzichi@matrix.com.br
Financial support: None.
Submitted: 26 March 2010. Accepted, after review: 22 July 2010.
About the authorsMichelle Gonçalves de Souza Tavares
Professor. Universidade do Sul de Santa Catarina - UNISUL, University of Southern Santa Catarina- Florianópolis, Brazil.
Márcia Margaret Menezes Pizzichini
Adjunct Professor. Department of Clinical Medicine, Universidade Federal de Santa Catarina - UFSC, Federal University of Santa Catarina - Florianópolis, Brazil.
Leila John Marques Steidle
Adjunct Professor. Department of Clinical Medicine, Universidade Federal de Santa Catarina - UFSC, Federal University of Santa Catarina - Florianópolis, Brazil.
Nazaré Otília Nazário
Professor. Universidade do Sul de Santa Catarina - UNISUL, University of Southern Santa Catarina- Florianópolis, Brazil.
Cristiane Cinara Rocha
Nurse. Núcleo de Pesquisa em Asma e Inflamação das Vias Aéreas - NUPAIVA, Center for Research on Asthma and Airway Inflammation - Polydoro Ernani de São Thiago University Hospital, Universidade Federal de Santa Catarina - UFSC, Federal University of Santa Catarina - Florianópolis, Brazil.
Maíra Chiaradia Perraro
Nurse. Núcleo de Pesquisa em Asma e Inflamação das Vias Aéreas - NUPAIVA, Center for Research on Asthma and Airway Inflammation - Polydoro Ernani de São Thiago University Hospital, Universidade Federal de Santa Catarina - UFSC, Federal University of Santa Catarina - Florianópolis, Brazil.
Emílio Pizzichini
Coordinator. Núcleo de Pesquisa em Asma e Inflamação das Vias Aéreas - NUPAIVA, Center for Research on Asthma and Airway Inflammation - Polydoro Ernani de São Thiago University Hospital, Universidade Federal de Santa Catarina - UFSC, Federal University of Santa Catarina - Florianópolis, Brazil.






