ABSTRACT
Objective: To study the incidence of nontuberculous mycobacteria and the range of species isolated between 1996 and 2005 at a regional branch of the Adolfo Lutz Institute-located in the city of São José do Rio Preto, Brazil-and to show the importance of laboratory testing. Methods: Mycobacteria were isolated from pulmonary and extrapulmonary specimens and identified through phenotyping and molecular methods (polymerase chain reaction-restriction enzyme analysis). Results: We isolated 317 nontuberculous mycobacterium strains: Mycobacterium avium complex, 182 (57.4%); M. gordonae, 33 (10.4%); M. fortuitum, 25 (7.9%); M. chelonae, 8 (2.5%); M. terrae complex, 8 (2.5%); M. kansasii, 7 (2.2%); and less frequent species, 54 (17%). During this period, 72 cases (33.3%) were characterized as mycobacteriosis, according to bacteriological criteria established by the American Thoracic Society in 2007. Of those 72 cases, 56 were attributed to M. avium complex. Of those 56, 29 (51.8%) were characterized as disseminated disease. Six cases were attributed to M. fortuitum, 3 to M. gordonae, 2 to M. chelonae, 1 to M. abscessus, 1 to M. kansasii, 1 to M. intracellulare, 1 to M. malmoense and 1 to Mycobacterium ssp. Conclusions: These results show the importance of the bacteriological diagnosis, since identification of the species enables early and appropriate treatment.
Keywords:
Mycobacteria, atypical/isolation & purification; Mycobacteria, atypical/classification; Diagnostic techniques and procedures.
RESUMO
Objetivo: Estudar a ocorrência de micobactérias não-tuberculosas e a variabilidade das espécies isoladas na região atendida pelo Instituto Adolfo Lutz-Regional de São José do Rio Preto-no período entre 1996 e 2005, assim como mostrar a importância do diagnóstico laboratorial. Métodos: A partir de amostras pulmonares e extrapulmonares, foi realizado o isolamento de micobactérias, e estas foram identificadas por métodos fenotípicos e pelo método molecular polymerase chain reaction-restriction enzyme analysis. Resultados: Foram isoladas 317 cepas de micobactérias não-tuberculosas: complexo Mycobacterium avium, 182 (57,4%); M. gordonae, 33 (10,4%); M. fortuitum, 25 (7,9%); M. chelonae, 8 (2,5%); complexo M. terrae, 8 (2,5%); M. kansasii, 7 (2,2%); e espécies menos freqüentes, 54 (17%). No período, foram caracterizados 72 casos (33,3%) de micobacterioses, de acordo com os critérios bacteriológicos estabelecidos pela American Thoracic Society (2007).Desses, complexo M. avium foi responsável por 56 casos, sendo que 29 (51,8%) foram caracterizados como doença disseminada. M. fortuitum foi responsável por 6 casos; M. gordonae, 3; M. chelonae, 2; M. abscessus, 1; M. kansasii, 1; M. intracellulare, 1; M. malmoense, 1; e Mycobacterium ssp., 1. Conclusões: Os resultados obtidos mostraram a importância do diagnóstico bacteriológico das micobacterioses, pois a identificação das espécies possibilita a introdução de um tratamento adequado precocemente.
Palavras-chave:
Micobactérias atípicas/isolamento & purificação; Micobactérias atípicas/classificação; Técnicas de diagnóstico e procedimentos.
IntroduçãoO gênero Mycobacterium é constituído pelo complexo Mycobacterium tuberculosis e outras espécies denominadas micobactérias não-tuberculosas (MNT). Nas últimas décadas, com o declínio da prevalência da tuberculose em países desenvolvidos, a proporção de doença por MNT está aumentando.(1) Entre estas, M. avium complex (MAC, complexo M. avium) emergiu como um importante patógeno humano, sendo a causa freqüente de doença disseminada e morte em pacientes portadores de HIV/AIDS.(2-5)
As micobactérias ocorrem naturalmente em diversos reservatórios: água natural e de sistemas de abastecimento, solo, aerossóis, protozoários, animais e humanos.(6-10) Existem poucas evidências da possibilidade de transmissão pessoa a pessoa, mas recentes relatos demonstraram a contaminação de pacientes devido à limpeza e desinfecção inadequada de equipamentos médicos.(5,11)
A doença pulmonar crônica é a manifestação clínica mais comum entre as doenças causadas por MNT, sendo as espécies pertencentes ao MAC, seguido de M. kansasii, os patógenos mais freqüentes. As características clínicas da doença pulmonar por MNT, em muitos casos, são extremamente semelhantes às da tuberculose. Outras manifestações clínicas podem ser causadas principalmente por M. fortuitum, M. chelonae e M. abscessus devido a infecções peritoneais por cateterização; infecções pós-cirúrgicas, como em mamoplastias e transplantes cardíacos; devido a procedimentos invasivos, como videoscopias, e devido a procedimentos estéticos. O patógeno mais freqüentemente isolado no caso de doença disseminada é o MAC.(2,9,11-13) Além desses eventos, há relatos mundiais do aumento da incidência de MNT observada pelo número de isolamentos realizados nos laboratórios, com conseqüente diversificação das espécies identificadas após o avanço das técnicas moleculares.(5,14,15)
Uma vez que o tratamento e a epidemiologia das micobacterioses diferem significantemente da tuberculose, a identificação correta e rápida destes organismos é obrigatória para o diagnóstico e esquema terapêutico corretos.(16)
O Instituto Adolfo Lutz - Regional de São José do Rio Preto (IAL-SJRP) é referência para o diagnóstico laboratorial da tuberculose da subdivisão XV (de Ribeirão Preto) da Divisão Regional de Saúde (DRS-XV) do estado de São Paulo e realiza as culturas de micobactérias, solicitadas pelos profissionais das Unidades de Saúde Pública que participam do Programa de Controle da Tuberculose da DRS-XV.
O objetivo deste trabalho foi estudar a ocorrência de MNT e a variabilidade das espécies isoladas na região atendida pelo IAL-SJRP no período entre 1996 e 2005, assim como mostrar a importância do diagnóstico laboratorial.
MétodosOs dados laboratoriais obtidos nos registros do IAL-SJRP no período entre janeiro/1996 e dezembro/2005, foram os seguintes: número de amostras analisadas, sítio de isolamento, número de culturas positivas e resultado da identificação das espécies. Foram recebidas uma ou mais amostras clínicas de sítios estéreis e não-estéreis por paciente. Os isolamentos foram obtidos de amostras pulmonares (escarro, lavado gástrico, lavado brônquico, secreção traqueal), ganglionares (linfonodos e secreção), pele (abscesso, secreção, ferimento), líquidos (peritoneal e pleural) e sangue. Esses foram processados segundo técnicas de isolamento padronizadas no manual técnico do Ministério da Saúde(17) e as culturas positivas foram encaminhadas ao IAL-Central para a identificação das espécies segundo métodos fenotípicos(8,18,19) e molecular.(14,15,20) As cepas foram submetidas a uma triagem pela análise macroscópica (morfologia e pigmento) e microscópica (presença de fator corda) da cultura. As culturas que apresentaram colônias acromógenas, rugosas e com presença de fator corda foram excluídas da amostragem. As cepas foram identificadas por métodos fenotípicos, de acordo com alguns autores,(4) os quais incluem a análise do tempo de crescimento pelos testes com ácido pícrico, nitrito de sódio, cloreto de sódio e ágar comum, temperatura de crescimento (26 °C, 37 °C e 45 °C), teste de fotocromogenicidade, testes bioquímicos (arilsulfatase-3 e 15 dias; β-galactosidase; atividade da nitrato-redutase, catalase e urease; utilização de açúcares; e hidrólise do Tween 80) e teste de crescimento na presença do ácido p-nitrobenzóico. A identificação molecular foi realizada, concomitantemente com a identificação fenotípica, pelo método polymerase chain reaction and restriction enzyme analysis - hsp65 (PRA-hsp65), modificado por nós em trabalho anterior,(21) baseado na amplificação de um fragmento de 441 pb do gene hsp65 e análise dos perfis de restrição gerados pelas enzimas BstEII e HaeIII, separadamente.
Este trabalho é parte do projeto "Estudo da diversidade de métodos moleculares de identificação de micobactérias em laboratórios públicos do estado de São Paulo", aprovado pelo Comitê de Ética do IAL em 2001 (CTC-BM 31/2001).
ResultadosNo período de 10 anos, foram isoladas, na região da DRS-XV, 1.300 culturas de micobactérias a partir de amostras clínicas analisadas no IAL-SJRP, sendo 983 isolamentos de (75,6%) M. tuberculosis e 317 (24,4%) de MNT (Tabela 1). A distribuição dos 317 isolamentos de MNT, provenientes de 216 pacientes, não foi homogênea neste período, sendo 2002 o ano com o maior índice, 81 cepas (44,8%), e 1999 com o menor índice, 13 cepas (9,7%).
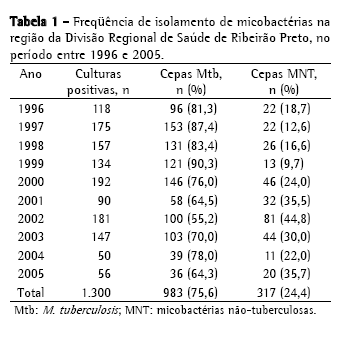
A Tabela 2 mostra a freqüência de espécies de MNT isoladas, sendo o MAC o mais freqüente, com 182 cepas (57,4%); seguido de M. gordonae, com 33 (10,4%); M. fortuitum, com 25 (7,9%); M. chelonae, com 8 (2,5%); complexo M. terrae, com 8 (2,5%); e M. kansasii, com 7 (2,2%). Foram identificadas 33 cepas (10,4%) de outras espécies de menor ocorrência; 20 cepas (6,3%) com identificação baseada somente no tempo de crescimento e pigmentação e 9 (2,8%) não tiveram identificação da espécie concluída, chegando somente à identificação do gênero. No período estudado, MAC foi a causa de doença em 56 pacientes, M. fortuitum em 6, M. gordonae em 3, M. chelonae em 2, M. abscessus em 1, M. kansasii em 1, M. intracellulare em 1, M. malmoense em 1 e micobactéria de crescimento rápido em 1 (Tabela 3). No total, 72 pacientes (33,3%) foram caracterizados como casos de micobacterioses, de acordo com os critérios bacteriológicos estabelecidos pela American Thoracic Society (2007).

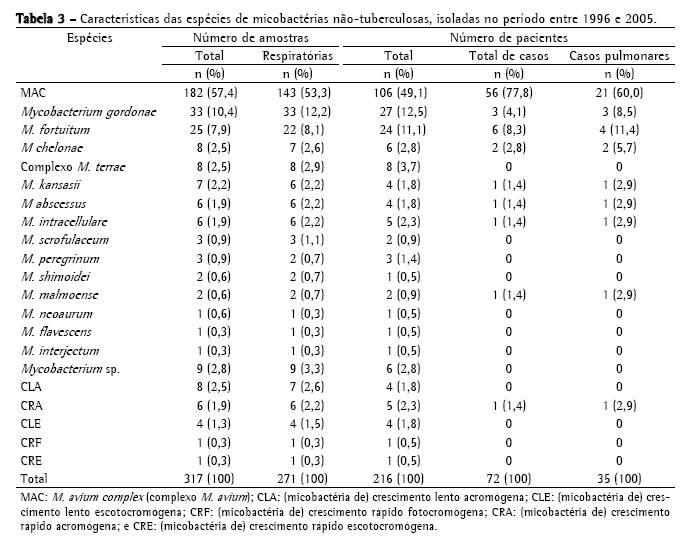
Os sítios de maior ocorrência de MAC neste período foram os pulmonares (78,6%). Dentre estes sítios, as amostras de escarro foram as mais freqüentes, com 132 cepas (73,2%) de MAC e 11 cepas (6%) isoladas de lavados gástricos e brônquicos. Entre os sítios estéreis, as amostras de sangue foram as mais freqüentes, com 28 isolamentos (15,4%).
A Tabela 4 apresenta a freqüência de pacientes com isolamento de MNT de sítios estéreis. A espécie mais freqüentemente isolada foi MAC, a partir de amostras de sangue, caracterizando doença disseminada em 24 pacientes (58,5%).
 Discussão
DiscussãoO número de espécies pertencentes ao gênero Mycobacterium dobrou nos últimos 15 anos, com muitas novas espécies clinicamente significantes.(10,22) Apesar de muitos trabalhos sugerirem que a incidência de micobacterioses tem aumentado nas últimas décadas, uma vez que estas doenças não são de notificação obrigatória no Brasil, esta observação não pode ser comprovada com registros oficiais.
A ocorrência de MNT (24,4%) na região estudada, no período de 10 anos, pode ser comparada com estudos do Hospital Universitário Clementino Fraga Filho (Rio de Janeiro): no período entre agosto de 1996 e julho de 1997, relatou-se 15% de MNT, e no período de 2000 a 2002, 45% de MNT.(23,24) Durante os mesmos períodos, o IAL-SJRP isolou 15% e 34,3% de MNT, respectivamente.
No Brasil, ainda é difícil comparar dados relativos às MNT, pois os poucos existentes são estudos isolados, com variação quanto ao período, ao tipo de amostras clínicas e à população analisada.(4) O aumento na freqüência de MNT a partir de 2001 pode ser explicado pela implantação do método automatizado com cultura líquida, método mais sensível que a cultura tradicional. A diminuição da demanda de culturas, a partir de 2004, ocorreu devido a uma interrupção parcial no serviço de identificação do IAL-Central e também pode ser atribuída à melhora dos pacientes atendidos nos centros especializados para DST/AIDS, com a introdução da highly active antiretroviral therapy (HAART, terapia anti-retroviral altamente ativa) na região. Apesar da diminuição do número de cepas analisadas no laboratório a partir de 2003, os números percentuais mostram que houve um aumento no isolamento de MNT.
Neste estudo, a identificação das espécies foi realizada no IAL-Central com testes fenotípicos até o ano de 2000 e posteriormente com método PRA-hsp65. As espécies M. avium e M. intracellulare puderam ser diferenciadas com a técnica molecular; na fase anterior, foram denominadas como MAC. Apesar do uso do termo MAC ser taxonomicamente aceito, ele esconde as características epidemiológicas únicas de cada espécie. O MAC (57,4%) foi o complexo com maior ocorrência, sendo 34,4% entre pacientes com AIDS. Após este período, tivemos ocorrência de 1,9% de M. intracellulare.
Segundo os critérios bacteriológicos estabelecidos pela American Thoracic Society, os isolamentos de MAC das amostras de 55 pacientes do presente estudo, permitiram a classificação bacteriológica de doença. De acordo com alguns autores,(25) que demonstraram situações onde pacientes assintomáticos apresentavam escarro positivo para MAC e alterações radiológicas, os dados bacteriológicos são de grande relevância para a caracterização de doença pulmonar por MAC. A disseminação hematogênica por MAC foi identificada em 24 (43,6%) destes pacientes.
A segunda maior ocorrência foi devido a M. gordonae (10,4%), normalmente considerada saprófita, que pode causar infecções pulmonares e cutâneas, principalmente em pacientes imunocomprometidos,(26) mas que também mostra a possibilidade de haver colonização transitória de MNT. M. fortuitum (7,9%) foi a terceira espécie mais freqüente, considerada potencialmente patogênica, responsável por muitos surtos recentemente descritos.(11)
Uma vez que as MNT e o homem ocupam os mesmos habitats, e devido ao crescente aumento de indivíduos suscetíveis às infecções por micobactérias (por ex., os imunossuprimidos), era provável que a prevalência das micobacterioses aumentasse.(27) Associado a este fato, novas tecnologias foram desenvolvidas com o objetivo de identificar esses agentes com maior eficiência e rapidez. Os dados obtidos neste estudo confirmam essas afirmações, pois antes da introdução do método molecular (ano 2000), eram identificadas somente as espécies contempladas nas tabelas fenotípicas. A partir desse período, não somente a freqüência de outras espécies aumentou, mas também o número de casos por outras MNT de importância clínica, como M. intracellulare, M. abscessus, M. chelonae e M. malmoense.
Devido à alta prevalência de tuberculose em nosso meio, o tratamento com drogas antituberculose tem início imediato após um resultado de exame indicativo de infecção por micobactérias. O tratamento das infecções por MNT depende das diferentes espécies e requer drogas alternativas, por longo período de tempo (de 18 a 24 meses). No estado de São Paulo, os casos devem ser registrados no Centro de Vigilância Epidemiológica-Divisão de Tuberculose para o fornecimento de drogas pelo Ministério da Saúde.
O presente estudo demonstrou a variabilidade das espécies de MNT na subdivisão da região de São José do Rio Preto e a importância clínica de determinadas espécies, cuja correta identificação foi fundamental no diagnóstico das micobacterioses. A instituição de um esquema adequado para determinadas espécies ainda é difícil, mas somente a elucidação desses casos possibilita o diagnóstico correto e uma estratégia apropriada de tratamento.
AgradecimentosAgradecemos o apoio do programa de capacitação do projeto Innovative approaches for tuberculosis control in Brazil do Programa International Clinical, Operational and Health Services Research and Training Award (ICOHRTA) AIDS/TB, do Fogarty International Center/National Institutes of Health dos
Estados Unidos.
Referências 1. Field SK, Cowie RL. Lung disease due to the more common nontuberculous mycobacteria. Chest. 2006;129(6):1653-72.
2. Murcia-Aranguren MI, Gómez-Marin JE, Alvarado FS, Bustillo JG, de Mendivelson E, Gómez B, et al. Frequency of tuberculous and non-tuberculous mycobacteria in HIV infected patients from Bogota, Colombia. BMC Infect Dis. 2001;1:21.
3. Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection. 2004;32(5):257-70.
4. Ueki SY, Martins MC, Telles MA, Virgilio MC, Giampaglia CM, Chimara E, et al. Micobactérias não tuberculosas: diversidade das espécies no estado de São Paulo. J Bras Patol Med Lab. 2005;41(1):1-8.
5. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
6. Martín-Casabona N, Bahrmand AR, Bennedsen J, Thomsen VO, Curcio M, Fauville-Dufaux M, et al. Non-tuberculous mycobacteria: patterns of isolation. A multi-country retrospective survey. Int J Tuberc Lung Dis. 2004;8(10):1186-93.
7. Covert TC, Rodgers MR, Reyes AL, Stelma GN Jr. Occurrence of nontuberculous mycobacteria in environmental samples. Appl Environ Microbiol. 1999;65(6):2492-6.
8. Morris A, Harrison AC. Non-tuberculosis mycobacteria. In: Ministry of Health. Guidelines for Tuberculosis Control in New Zealand 2003. Wellington: Ministry of Health, 2002.
9. Morrone N, Cruvinel MC, Morrone Jr N, Freire JA, Oliveira LM, Gonçalves C. Pneumopatia causada por Mycobacterium kansasii. J Pneumol. 2003;29(6):341-49.
10. Primm TP, Lucero CA, Falkinham JO 3rd. Health impacts of environmental mycobacteria. Clin Microbiol Rev. 2004;17(1):98-106.
11. Padoveze MC, Fortaleza CM, Freire MP, Brandão de Assis D, Madalosso G, Pellini AC, et al. Outbreak of surgical infection caused by non-tuberculous mycobacteria in breast implants in Brazil. J Hosp Infect. 2007;67(2):161-7.
12. Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005;20(6):913-25.
13. Koh WJ, Kwon OJ, Lee KS. Nontuberculous mycobacterial pulmonary diseases in immunocompetent patients. Korean J Radiol. 2002;3(3):145-57.
14. Brunello F, Ligozzi M, Cristelli E, Bonora S, Tortoli E, Fontana R. Identification of 54 mycobacterial species by PCR-restriction fragment length polymorphism analysis of the hsp65 gene. J Clin Microbiol. 2001;39(8):2799-806.
15. Telenti A, Marchesi F, Balz M, Bally F, Böttger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31(2):175-8.
16. Secretaria Estadual da Saúde. Coordenadoria de Controle de Doenças. Micobacterioses: recomendações para o diagnóstico e tratamento. São Paulo: Secretaria Estadual da Saúde de São Paulo; 2005.
17. Brasil. Ministério da Saúde. Programa Nacional de Combate da Tuberculose. Manual de Bacteriologia da Tuberculose. Rio de Janeiro: Fundação Nacional da Saúde, 2005.
18. Kent PT, Kubica GP. Public health mycobacteriology: a guide for the level III laboratory. Atlanta: U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control; 1985.
19. Tsukamura M. Identification of mycobacteria. Obui: The National Chubu Hospital, Japan; 1984.
20. Chimara E, Giampaglia CM, Martins MC, Telles MA, Ueki SY, Ferrazoli L. Molecular characterization of Mycobacterium kansasii isolates in the State of São Paulo between 1995-1998. Mem Inst Oswaldo Cruz. 2004;99(7):739-43.
21. Chimara E, Ferrazoli L, Ueky SY, Martins MC, Durham AM, Arbeit RD, et al. Reliable identification of mycobacterial species by PCR-restriction enzyme analysis (PRA)-hsp65 in a reference laboratory and elaboration of a sequence-based extended algorithm of PRA-hsp65 patterns. BMC Microbiol. 2008;8:48.
22. Hall L, Roberts GD. Non-molecular identification of nontuberculous mycobacteria in the clinical microbiology laboratory: What's the real deal? Clin Microbiol News. 2006;28(10):73-80.
23. Ferreira RM, Saad MH, Silva MG, Fonseca LS. Non-tuberculous mycobacteria I: one year clinical isolates identification in Tertiary Hospital Aids Reference Center, Rio de Janeiro, Brazil, in pre highly active antiretroviral therapy era. Mem Inst Oswaldo Cruz. 2002;97(5):725-9.
24. Senna SG, Marsico AG, Suffys PN, Fonseca LS. Identificação e análise de MNTs causadoras de infecção no Hospital Universitário - HUCFF/UFRJ no Rio de Janeiro. J Bras Pneumol. 2006;32 (Supl 3):S135-S158.
25. Ye JJ, Wu TS, Chiang PC, Lee MH. Factors that affect sputum conversion and treatment outcome in patients with Mycobacterium avium-intracellulare complex pulmonary disease. J Microbiol Immunol Infect. 2007;40(4):342-8.
26. Weinberger M, Berg SL, Feuerstein IM, Pizzo PA, Witebsky FG. Disseminated infection with Mycobacterium gordonae: report of a case and critical review of the literature. Clin Infect Dis. 1992;14(6):1229-39.
27. Falkinham JO. The changing pattern of nontuberculous mycobacterial disease. Can J Infect Dis. 2003;14(5):281-6.
Trabalho realizado no Instituto Adolfo Lutz, Regional de São José do Rio Preto, São José do Rio Preto (SP) Brasil.
1. Pesquisadora científica. Instituto Adolfo Lutz, Regional de São José do Rio Preto, São José do Rio Preto (SP) Brasil.
2. Assistente de Apoio em Pesquisa Científica e Tecnológica. Instituto Adolfo Lutz, Regional de São José do Rio Preto, São José do Rio Preto (SP) Brasil.
3. Biologista. Instituto Adolfo Lutz, Regional de São José do Rio Preto, São José do Rio Preto (SP) Brasil.
Endereço para correspondência: Erica Chimara. Av. Dr. Arnaldo, 351, 9º andar, CEP 01246-902, São Paulo, SP, Brasil.
4. Pesquisadora científica. Instituto Adolfo Lutz, Central, São Paulo, Brasil
Tel 55 17 55 11 30682895. E-mail: echimara@ial.sp.gov.br
Apoio financeiro: Nenhum.
Recebido para publicação em 3/3/2008. Aprovado, após revisão, em 31/3/2008.





