ABSTRACT
Objective: To describe the characteristics of patients with influenza A (H1N1)-associated pneumonia treated at two hospitals in the region of Liguria, Italy, as well as to describe their treatment and outcomes. Methods: This was a prospective observational study including all patients older than 16 years of age with a confirmed diagnosis of influenza A (H1N1) who were admitted to Villa Scassi Hospital, in the city of Genoa, Italy, or to the Sestri Levante General Hospital, in the city of Sestri Levante, Italy, between September of 2009 and January of 2010. The primary outcome measure was mortality within 60 days after diagnosis. Secondary outcome measures were the need for mechanical ventilation and the length of hospital stay. Results: Of the 40 patients with a confirmed diagnosis of influenza A (H1N1), 27 presented pneumonia during the study period. The mean age of the 27 patients was 42.8 ± 14.8 years, and the mean length of hospital stay was 11.6 ± 8.2 days. Of the 27 patients, 20 had respiratory failure, 4 underwent invasive mechanical ventilation, and 5 underwent noninvasive ventilation. One patient had comorbidities, developed multiple organ failure, and died. Conclusions: During the influenza A (H1N1) pandemic, the associated mortality rate was lower in Italy than in other countries, and cases reported in the country typically had a milder course than did those reported elsewhere. Nevertheless, 9 of our cases (33%) rapidly evolved to respiratory failure, requiring mechanical ventilation.
Keywords:
Pneumonia; Influenza A virus, H1N1 subtype; Respiratory insufficiency.
RESUMO
Objetivo: Descrever as características dos pacientes com pneumonia associada a influenza A (H1N1) tratados em dois hospitais na região da Ligúria, Itália, e descrever seu tratamento e desfechos. Métodos: Estudo prospectivo observacional que incluiu todos os pacientes com mais de 16 anos de idade e com diagnóstico confirmado de influenza A (H1N1) admitidos no Hospital Villa Scassi, em Gênova, ou no Hospital Geral de Sestri Levante, em Sestri Levante, Itália, entre setembro de 2009 e janeiro de 2010. O desfecho primário foi mortalidade em até 60 dias do diagnóstico, e os desfechos secundários foram necessidade de ventilação mecânica e tempo de hospitalização. Resultados: Durante o período do estudo, dos 40 pacientes com diagnóstico confirmado de influenza A (H1N1), 27 apresentaram pneumonia. A média de idade dos 27 pacientes foi de 42,8 ± 14,8 anos, e o tempo médio de hospitalização foi de 11,6 ± 8,2 dias. Dos 27 pacientes, 20 tiveram insuficiência respiratória, 4 necessitaram de ventilação mecânica invasiva e 5, de ventilação mecânica não invasiva. Somente 1 paciente com várias comorbidades teve falência múltipla de órgãos e faleceu. Conclusões: Embora a influenza A (H1N1) tenha sido mais branda e com menor incidência de mortalidade na Itália do que em outros países, 9 de nossos pacientes (33%) tiveram evolução rápida para falência respiratória e necessitaram de ventilação mecânica.
Palavras-chave:
Pneumonia; Vírus da influenza A subtipo H1N1; Insuficiência respiratória.
IntroductionNew diseases pose a challenge to clinicians. When a novel infectious disease, influenza A (H1N1), became pandemic, it caused severe illness and resulted in significant increases in the utilization of health care services worldwide.(1,2) The incubation period of the disease was similar to that of seasonal flu.(3) Approximately a quarter of all patients with this pandemic flu presented with gastrointestinal symptoms,(4,5) and approximately 40% of all hospitalized patients had findings consistent with pneumonia on initial chest X-rays. In addition, 10-30% of hospitalized patients required admission to ICUs and mechanical ventilation.(3,6,7) Radiographic findings were similar to those seen in cases of severe pneumonia.(4,8) Viral specimens from the lower respiratory tract (BAL fluid samples) are more reliable than are those of samples from the upper airway.(9) Rapid antigen tests have lower sensitivity and cannot exclude the diagnosis. The preferred test is real-time PCR, which has a sensitivity of 98%, a positive predictive value of 100%, and a negative predictive value of 98%.(9,10) Antiviral drugs are recommended only for high-risk patients or severe cases. When secondary bacterial pneumonia is suspected, antibiotics must be used.(11) Other treatment modalities include ventilatory support and, in cases of severe pneumonia, corticosteroid therapy.(3) The major complications are respiratory failure and acute respiratory distress syndrome (ARDS).(3,4,6) Autopsies of these patients have shown extensive diffuse alveolar damage, pulmonary hemorrhage, and necrotizing bronchiolitis.(12,13) Higher Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, lower PaO2/FiO2 ratio, shock, hemodialysis, and Streptococcus pneumoniae infection are independent factors predicting death.(6,7,10,14)
From the beginning of the epidemic, influenza A (H1N1) infection seemed to have a more severe course and worse outcomes than did infection with seasonal influenza A. In addition, the demographic profile of influenza A (H1N1) infection was younger, and it affected individuals with fewer comorbidities. More severe respiratory involvement was noted, and a greater number of patients were admitted to ICUs with influenza A (H1N1)-associated pneumonia. The objective of the present study was to describe the characteristics of the patients with influenza A (H1N1)-associated pneumonia treated at two hospitals in the region of Liguria, Italy, between September of 2009 and January of 2010, as well as to describe their treatment and outcomes.
Methods Between September of 2009 (when the first case was diagnosed in the Italian region of Liguria) and January of 2010, a prospective observational study was conducted in Villa Scassi Hospital, Genoa, and in the General Hospital of Sestri Levante, Sestri Levante, Italy. The inclusion criteria were being at least 16 years of age, being diagnosed with influenza A (H1N1) infection, and presenting with radiological findings suggestive of pneumonia. The exclusion criteria were being hospitalized in the preceding 10 days and having concurrent lung cancer or tuberculosis. The primary outcome measure was mortality within 60 days after diagnosis. Secondary outcomes included the use of mechanical ventilation and ICU admission. The study design was approved by the research ethics committees of the institutions.
During the study period, 40 patients diagnosed with influenza A (H1N1) were admitted to one of the hospitals. The diagnosis was confirmed by reverse transcriptase PCR (performed at the Institute for Public Health of the Medical University of Genoa, Genoa, Italy), and all tests were carried out using the guidelines recommended by the U.S. Centers for Disease Control and Prevention.(6) Nasopharyngeal swab specimens were collected from all of the patients at admission, and bronchial aspirate samples were obtained after tracheal intubation from the patients who required intubation. From a patient with suspected oseltamivir-resistant infection, BAL fluid was collected. In order to stratify the patients at admission, severity of illness was assessed using APACHE II score, the Simplified Acute Physiology Score II, and the PaO2/FiO2 ratio (during oxygen delivery with a high-flow face mask).(15) All patients were placed in negative-pressure isolation rooms.
Of the 40 patients, 27 presented with pneumonia. Any patient meeting the criteria for influenza A (H1N1) infection and evidence of recent pulmonary infiltrate on chest X-rays, without an alternative diagnosis, was classified as having pneumonia. Chest X-rays were independently evaluated by two investigators. We also used an adapted score system (chest X-ray score) in order to grade the radiological severity of the pulmonary infiltrates.(15) Any patient meeting the criteria for influenza A (H1N1)-associated pneumonia with a positive culture for a bacterial pathogen from blood or BAL fluid samples was considered to have influenza (H1N1)-associated pneumonia and a bacterial co-infection. A chest CT scan was performed in the case of inconclusive chest X-ray findings or in order to evaluate the extent of pneumonia.
Statistical analysis was carried out dividing the patients into two groups: patients with mild disease and patients with severe disease. The latter group comprised patients with sepsis, shock, acute lung injury, or ARDS, as well as patients who required intubation or noninvasive mechanical ventilation and patients admitted to the ICU or to the respiratory intermediate care unit.(16) Acute lung injury was defined as a PaO2/FiO2 ratio between 201 and 300, whereas ARDS was defined as a PaO2/FiO2 ratio ≤ 200.(17) Baseline clinical and laboratory characteristics of the two groups were compared using logistic regression analysis for categorical variables and analysis of covariance for continuous variables. The level of statistical significance was set at p < 0.05.
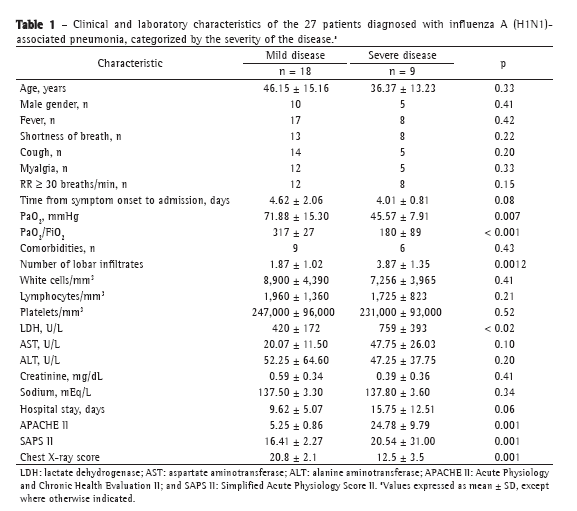
ResultsData related to the 27 patients with confirmed influenza A (H1N1)-associated pneumonia were analyzed. At admission, all patients were appropriately treated with oseltamivir (75 mg, twice a day) and received empirical treatment with antibiotics: 18 patients (66%) were treated with ceftriaxone, 3 (12%) received the ceftriaxone-clarithromycin combination, and 6 (22%) received additional broad-spectrum antibiotics. Bacterial co-infection (with Staphylococcus aureus) was found in only 1 patient (in a BAL fluid sample). Clinical and laboratory characteristics of the 27 patients are reported in Table 1. Approximately 75% of the hospitalized patients were between 18 and 51 years of age. The male-to-female ratio was 1.25:1.00. The mean length of hospital stay was 11.6 ± 8.2 days; the number of days from the onset of influenza-like symptoms to hospital admission and initiation of antiviral therapy was 4.28 ± 1.72 days. During hospitalization, respiratory failure was documented in 19 of the 27 patients (70%), and ARDS was identified in 9 of those patients (33%). Of the 9 patients who required mechanical ventilation, 4 received invasive mechanical ventilation. One patient (3.7%), who suffered from dementia, developed multiple organ failure and died. That was the only patient who required extracorporeal membrane oxygenation. Fifteen patients had underlying medical conditions: bronchial asthma, in 4 (26.0%); COPD, in 3 (20.0%); diabetes, in 2 (13.5%); obesity, in 2 (13.5%); dementia, in 3 (20.0%); and hairy cell leukemia, in 1 (7%). Of the patients admitted to the ICU or to the respiratory intermediate care unit, 6 presented with comorbidities: dementia, in 2 (33.0%); COPD, in 2 (33.0%); diabetes, in 1 (16.5%); and obesity, in 1 (16.5%). The patients with dementia had the worst outcomes: 1 died, and another underwent intubation and had the longest stay in the ICU. The characteristics of the 9 patients admitted to the ICU or to the respiratory intermediate care unit are described in Table 2. In the comparison of the two groups (mild disease vs. severe disease), there were no significant differences regarding the mean age of the patients and the mean number of days from the onset of symptoms to the initiation of antiviral therapy. Higher levels of lactate dehydrogenase (LDH), higher PaO2, higher PaO2/FiO2 ratio, higher chest X-ray scores, and greater number of lobes affected by the disease were independent variables that were associated with mechanical ventilation requirement and ICU admission, but not with 60-day mortality (Figure 1).
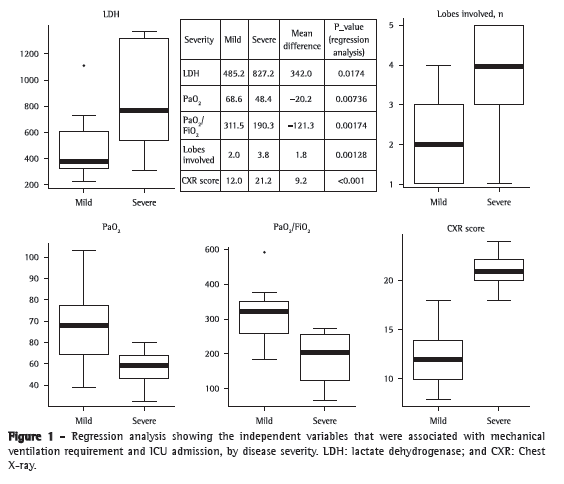
 Discussion
DiscussionWhen the influenza A (H1N1) virus first appeared, it was evident that it could cause severe illness,(6) and this was confirmed in various studies.(6,9,12,14-28) Approximately 20-56% of the patients hospitalized with influenza A (H1N1) infection had respiratory failure and required mechanical ventilation.(6,18,19,25) The risk of developing respiratory failure has been significantly associated with scores, at admission, of ≥ 4 on the Sequential Organ Failure Assessment or ≥ 20 on the APACHE II, as well as with lymphocyte counts ≤ 800 mm3, interval between symptom onset and initiation of antiviral therapy > 48 h, chest X-ray scores ≥ 12,(21,25) and a body mass index > 40 kg/m2.(26) Influenza A (H1N1)-associated ARDS remains uncommon.(27) The most common diagnosis was pneumonia,(6,18,19) and the majority of pneumonia-related deaths occurred in young healthy adults with comorbidities.(9,26) In Europe (including Italy), influenza A (H1N1) infection had a milder course than in other countries.(28) However, among the severe cases evaluated in the present study, we identified respiratory failure and the need for mechanical ventilation in 19 (70%) and 9 (33%), respectively, findings that are in agreement with those of other authors.(6,8,9,18,19) All of our patients were strictly monitored for the risk of rapid deterioration, especially for increased oxygen demand.(24) Although secondary outcomes, such as the frequency of mechanical ventilation requirement and length of hospital stay, were similar to those reported for other countries, mortality was lower in our study than that in previous studies of the pandemic. In our study, age and gender distributions were similar to those previously reported.(9,28,29) Nearly 60% of the patients had pre-existing medical conditions, chronic lung diseases, diabetes, and hypertension being the most common, as has previously been reported.(6,29) In our patients, symptoms included fever, cough, myalgia, and dyspnea, which have often been reported in other studies. However, the incidence of gastrointestinal symptoms, such as nausea, vomiting, and diarrhea, was much lower than in previous reports.(29) We observed elevated transaminase and LDH levels, which is also consistent with other reports.(6,29) Elevated LDH has been found to be significantly associated with the severity of the illness and admission to ICU.(29) In our sample, 33% of the patients were admitted to the ICU. There was only one death. Although our data regarding ICU admission are similar to those collected in other countries,(29) mortality was lower in our study. Another study described patients with influenza A (H1N1) infection who were admitted to four infectious disease facilities in Liguria.(30) That study included 81 patients, of whom 50% had pneumonia, 12% were admitted to ICU, and 3% died. The authors found that respiratory and neurocognitive impairment was associated with severe disease and death. In our study, the only patient who died suffered from a neurocognitive disorder. To our knowledge, this association had not been previously described in the literature in English.
One limitation of our study is that the study samples represented less than 1% of all reported hospitalizations for influenza A (H1N1)-associated pneumonia in Italy. Our hospitals are not referral centers for children and pregnant woman infected with influenza A (H1N1). Therefore, no children or pregnant women were included in our study. This was an observational study, and participation was voluntary. It included only patients with confirmed influenza A (H1N1) infection. Therefore, our sample might not be representative of all hospitalized patients. Finally, the low mortality rate in our sample makes it impossible to compare our study with other studies or countries in terms of this outcome (mortality).
In conclusion, patients with suspected influenza A (H1N1) infection should be moved to negative-pressure isolation rooms as soon as possible to avoid transmission of the infection. They should receive continuous oxygen monitoring. Antiviral treatment should not be delayed. This infection requires proactive management.
References1. Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361(7):674-9.
2. Satpathy HK, Lindsay M, Kawwass JF. Novel H1N1 virus infection and pregnancy. Postgrad Med. 2009;121(6):106-12.
3. Marjani M, Parvaneh B, Tabarsi P, Mansouri SD. Update on 2009 pandemic Influenza A (H1N1) virus. Tanaffos. 2010;9(1):8-14.
4. Gordon SM. Update on 2009 pandemic influenza A (H1N1) virus. Cleve Clin J Med. 2009;76(10):577-82.
5. Zimmer SM, Burke DS. Historical perspective--Emergence of influenza A (H1N1) viruses. N Engl J Med. 2009;361(3):279-85.
6. Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680-9.
7. Bewick T, Myles P, Greenwood S, Nguyen-Van-Tam JS, Brett SJ, Semple MG, et al. Clinical and laboratory features distinguishing pandemic H1N1 influenza-related pneumonia from interpandemic community-acquired pneumonia in adults. Thorax. 2011;66(3):247-52.
8. Marchiori E, Zanetti G, Hochhegger B, Rodrigues RS, Fontes CA, Nobre LF, et al. High-resolution computed tomography findings from adult patients with
Influenza A (H1N1) virus-associated pneumonia. Eur J Radiol. 2010;74(1):93-8.
9. Blyth CC, Iredell JR, Dwyer DE. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;361(25):2493.
10. Cunha BA. Swine Influenza (H1N1) pneumonia: clinical considerations. Infect Dis Clin North Am. 2010;24(1):203-28.
11. Barlow GD; BSAC Council. Swine flu and antibiotics. J Antimicrob Chemother. 2009;64(5):889-94.
12. Mauad T, Hajjar LA, Callegari GD, da Silva LF, Schout D, Galas FR, et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med. 2010;181(1):72-9.
13. Mukhopadhyay S, Philip AT, Stoppacher R. Pathologic findings in novel influenza A (H1N1) virus ("Swine Flu") infection: contrasting clinical manifestations and lung pathology in two fatal cases. Am J Clin Pathol. 2010;133(3):380-7.
14. Chien YS, Su CP, Tsai HT, Huang AS, Lien CE, Hung MN, et al. Predictors and outcomes of respiratory failure among hospitalized pneumonia patients with 2009 H1N1 influenza in Taiwan. J Infect. 2010;60(2):168-74.
15. Kute VB, Godara SM, Goplani KR, Gumber MR, Shah PR, Vanikar AV, et al. High mortality in critically ill patients infected with 2009 pandemic influenza A (H1N1) with pneumonia and acute kidney injury. Saudi J Kidney Dis Transpl. 2011;22(1):83-9.
16. Zarychanski R, Stuart TL, Kumar A, Doucette S, Elliott L, Kettner J, et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182(3):257-64.
17. Gómez-Gómez A, Magaña-Aquino M, Garcia-Sepúlveda C, Ochoa-Pérez UR, Falcón-Escobedo R, Comas-García A, et al. Severe pneumonia associated with pandemic (H1N1) 2009 outbreak, San Luis Potosí, Mexico. Emerg Infect Dis. 2010;16(1):27-34.
18. Riquelme R, Riquelme M, Rioseco ML, Inzunza C, Gomez Y, Contreras C, et al. Characteristics of hospitalised patients with 2009 H1N1 influenza in Chile. Eur Respir J. 2010;36(4):864-9.
19. Estenssoro E, Ríos FG, Apezteguía C, Reina R, Neira J, Ceraso DH, et al. Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010;182(1):41-8.
20. Xiao H, Lu SH, Ou Q, Chen YY, Huang SP. Hospitalized patients with novel influenza A (H1N1) virus infection: Shanghai, June - July 2009. Chin Med J (Engl). 2010;123(4):401-5.
21. Koegelenberg CF, Irusen EM, Cooper R, Diacon AH, Taljaard JJ, Mowlana A, et al. High mortality from respiratory failure secondary to swine-origin influenza A (H1N1) in South Africa. QJM. 2010;103(5):319-25.
22. Choi WJ, Kim WY, Kim SH, Oh BJ, Kim W, Lim KS, et al. Clinical characteristics of pneumonia in hospitalized patients with novel influenza A (H1N1) in Korea. Scand J Infect Dis. 2010;42(4):311-4.
23. Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896-902.
24. Rello J, Rodríguez A, Ibañez P, Socias L, Cebrian J, Marques A, et al. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13(5):R148.
25. Chien YS, Su CP, Tsai HT, Huang AS, Lien CE, Hung MN, et al. Predictors and outcomes of respiratory failure among hospitalized pneumonia patients with 2009 H1N1 influenza in Taiwan. J Infect. 2010;60(2):168-74.
26. Gill JR, Sheng ZM, Ely SF, Guinee DG, Beasley MB, Suh J, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134(2):235-43.
27. Lai AR, Keet K, Yong CM, Diaz JV. Severe H1N1-Associated acute respiratory distress syndrome: A case series. Am J Med. 2010;123(3):282-285.
28. Cantero Caballero M, Touma Fernández A, Granda Martín MJ, Castuera Gil A, Zegarra Salas P, Cuenca Carvajal C, et al. Hospital care of patients with A/H1N1 influenza: evaluation of the first 1000 reported cases in Spain [Article in Spanish]. Med Clin (Barc). 2010;135(1):1-7.
29. Cui W, Zhao H, Lu X, Wen Y, Zhou Y, Deng B, et al. Factors associated with death in hospitalized pneumonia patients with 2009 H1N1 influenza in Shenyang, China. BMC Infect Dis. 2010;10:145.
30. Bassetti M, Parisini A, Calzi A, Pallavicini FM, Cassola G, Artioli S, et al. Risk factors for severe complications of the novel influenza A (H1N1): analysis of patients hospitalized in Italy. Clin Microbiol Infect. 2011;17(2):247-50.
* Study carried out at the Villa Scassi Hospital, Genoa, Italy, and at the Sestri Levante General Hospital, Sestri Levante, Italy.
Correspondence to: Antonello Nicolini. Ospedale Civile Sestri Levante, Via Arnaldo Terzi, 43, 16039, Sestri Levante, GE, Itália.
Tel. 39 018541031. E-mail: antonello.nicolini@fastwebnet.it
Financial support: None.
Submitted: 11 September 2010. Accepted, after review, 19 July 2011.
**A versão completa em português deste artigo está disponível em www.jornaldepneumologia.com.brOriginal
About the authorsAntonello Nicolini
Physician. Division of Respiratory Diseases, General Hospital of Sestri Levante, Sestri Levante, Italy.
Simonassi Claudio
Physician. Division of Respiratory Diseases, Villa Scassi Hospital, Genoa, Italy.
Fabrizio Rao
Physician. Division of Respiratory Diseases, Villa Scassi Hospital, Genoa, Italy.
Lorenzo Ferrera
Physician. Division of Respiratory Diseases, Villa Scassi Hospital, Genoa, Italy.
Michele Isetta
Physician. Division of Respiratory Diseases, Villa Scassi Hospital, Genoa, Italy.
Monica Bonfiglio
Physician. Intensive Care Unit, Lavagna Hospital, Lavagna, Italy.




