ABSTRACT
In humans, the most common types of infection are respiratory tract infections, among which viral infections predominate. Viruses can also infect the low respiratory tract, causing bronchiolitis, bronchitis and pneumonia. The objective of this review article was to show epidemiological, pathophysiological, clinical and therapeutic aspects of viral community-acquired pneumonia. These types of pneumonia are commonly caused by influenza A and B; parainfluenza 1, 2 and 3; respiratory syncytial virus; or adenovirus. We also address the types of pneumonia caused by hantaviruses, metapneumoviruses and rhinoviruses.
Keywords:
Pneumonia, viral; Influenza, human; Respiratory syncytial virus infections; Hantavirus.
RESUMO
As infecções do trato respiratório são as formas de infecção mais comuns que afetam o homem e, dentre essas, predominam as de causa viral. Os vírus também podem acometer o trato respiratório baixo, causando bronquiolite, bronquite e pneumonia. Neste artigo de revisão, objetivamos mostrar aspectos epidemiológicos, fisiopatológicos, clínicos e do tratamento das pneumonias comunitárias por vírus. Essas pneumonias costumam ser causadas por vírus influenza A e B; parainfluenza 1, 2 e 3; vírus respiratório sincicial; e adenovírus. Também são apresentados aqui os hantavírus, metapneumovírus e rinovírus causando pneumonia.
Palavras-chave:
Pneumonia viral; Influenza humana; Infecções por vírus respiratório sincicial; Hantavírus.
As infecções do trato respiratório são as formas de infecção mais comuns que afetam o homem e, dentre essas, predominam as de causa viral. Sabe-se hoje da existência de 1.200 vírus que infectam o trato respiratório, embora muitos deles, provavelmente, não causem doença.(1) Como consequência de infecções do trato respiratório por vírus, destacam-se duas doenças muito comuns, mas de difícil distinção: o resfriado, que tem pequena gravidade e duração, cursando com cefaleia, espirros, calafrios e dor de garganta, evoluindo com coriza, obstrução nasal, tosse e mal-estar; e a gripe, mais grave, de instalação súbita com febre, cefaleia, tosse, dor de garganta, mialgia, espirros, fraqueza e hiporexia. Entretanto, os vírus também podem acometer o trato respiratório baixo, causando bronquiolite, bronquite e pneumonia.(2)
Define-se pneumonia viral como aquele acometimento em que ocorre anormalidade nas trocas gasosas a nível alveolar, acompanhada por inflamação do parênquima pulmonar. O fenômeno inflamatório do pulmão, comumente, traduz-se em anormalidades de imagem detectáveis por radiografia ou TC. Nas pneumonias virais, os quadros clínicos são muito variáveis, dependendo do agente infectante, bem como da idade e do estado imune do hospedeiro. Nos últimos anos, ganharam notoriedade como causadores de graves pneumonias, que levam à insuficiência respiratória e alta letalidade, os coronavírus da severe acute resporatory syndrome (SARS, síndrome respiratória aguda grave), os vírus influenza A tipo H5N1 (da gripe aviária) e os hantavírus americanos.(1) Por outro lado, além da pneumonia primária, os vírus, ao lesarem a mucosa do trato respiratório, prejudicam seus mecanismos locais de defesa, favorecendo assim o surgimento de pneumonias bacterianas secundárias. Além disso, algumas doenças crônicas, como a DPOC, a insuficiência cardíaca e mesmo a gravidez têm sido descritas como associadas a um maior risco de pneumonias por vírus.(3)
As pneumonias virais podem ser consequentes de infecções que se originam no próprio trato respiratório, progredindo, por contiguidade ou por contaminação através de aerossóis, até atingirem o bronquíolo terminal - como, por exemplo, influenza e respiratory syncytial virus (RSV, vírus respiratório sincicial) - ou de infecções originárias do trato respiratório que se disseminam sistemicamente e, como consequência, o patógeno atinge o trato respiratório baixo (por ex: sarampo), ou ainda, a infecções originárias de outros locais que, por via sistêmica, atingem o referido trato (por ex: citomegalovírus). Estudos sobre a patogênese da pneumonia por influenza mostram que o vírus, em casos de diferentes gravidades, acomete pneumócitos como alvo primário, com dano alveolar difuso. A submucosa apresenta-se hiperêmica, com hemorragias focais, edema e infiltrado celular associado ao processo intra-alveolar, contendo neutrófilos, células mononucleares com fibrina e fluído edematoso. Em uma fase mais avançada da infecção, ocorre organização fibrocelular intra-alveolar (bronquiolite obliterante com pneumonia em organização) com presença de histiócitos e pneumócitos multinucleados.(3,4) No epitélio respiratório, a imunidade humoral contra os vírus influenza mostra-se com os anticorpos neutralizantes. Quanto à resposta imune celular, linfócitos T CD4 atraem células imunes em resposta do tipo MHC classe II, e também linfócitos T CD8 citotóxicos induzem resposta tipo MHC classe I, com produção de IFN-γ e TNF, induzindo a lise de células infectadas e apoptose.(5) Na síndrome pulmonar e cardiovascular por hantavírus (SPCVH), a pneumonite intersticial, o edema pulmonar e o choque cardiogênico, que caracterizam a doença, ocorrem, em grande parte, em virtude da ativação da resposta imune, especialmente de linfócitos T CD8+ e macrófagos, ambos produtores de citocinas inflamatórias. Embora as células endoteliais, alvos da infecção viral, produzam uma resposta antiviral de IFN, muitas espécies de hantavírus podem inibir ou retardar ativamente tais respostas. Além disso, os hantavírus exercem um efeito inibidor sobre os receptores celulares responsáveis pela manutenção da integridade vascular. Quadros clínicos de maior gravidade também têm sido associados a elevadas cargas virais. Inversamente, anticorpos neutralizantes parecem exercer um efeito protetor contra as formas graves.(6) Ainda, na pneumonia grave por RSV, tem sido observada intensa replicação viral local, resposta pró-inflamatória exacerbada e um alto nível de ativação das células T.(7)
Não existem critérios clínicos em pacientes com pneumonia que sugiram, com absoluta segurança, a etiologia viral. Além disso, existem dificuldades no diagnóstico etiológico geral das pneumonias e, particularmente, no daquelas por vírus, o que limita o conhecimento sobre essas doenças e sobre seus agentes causadores. A descrição clássica de uma pneumonia viral grave baseia-se naquela descrita para o vírus influenza A. Os pacientes apresentam tosse, inicialmente não produtiva, mas que pode evoluir com produção de escarro mucoso róseo, e insuficiência respiratória, caracterizada por cianose e hipóxia. Ao exame, o paciente apresenta-se agudamente enfermo e, eventualmente, com rinite e conjuntivite, exibindo um aumento da frequência respiratória e crepitações disseminadas pela zona de projeção do parênquima pulmonar. Nas pneumonias por RSV, ganham importância a bronquite e a bronquiolite associadas à lesão necrótica de mucosa e ao arrolhamento por muco.(3) Em adultos, febre alta, leucocitose com neutrofilia e imagem radiológica de pneumonia lobar não predizem a etiologia viral. Da mesma forma, em crianças, analisaram-se alterações clínicas e de exames complementares, tais como, temperatura axilar > 39°C, exame hematológico com contagem total de neutrófilos > 8.000/mm3 de sangue e exame radiológico exibindo infiltrado pulmonar bem definido, lobar ou segmentar. Essas alterações clínicas e de exames complementares foram organizadas de modo a, quando presentes, produzir notas cuja somatória, quando elevada, indicaria uma alta probabilidade de pneumonia bacteriana. Por outro lado, uma nota baixa (menor que 4) seria uma predição para a etiologia viral em casos infantis de pneumonia.(8)
Tradicionalmente, os laboratórios de virologia utilizam, no diagnóstico das infecções respiratórias, sangue e swabs ou aspirados das secreções de nasofaringe e orofaringe, os quais são processados para o isolamento viral em cultura de células, para o uso em métodos sorológicos, com detecção de anticorpos IgM, sugestivos de infecção recente, assim como em métodos imunoenzimáticos, para a detecção de IgG. Entretanto, nos últimos 15 anos, popularizaram-se técnicas altamente sensíveis para a detecção genômica de patógenos em materiais clínicos, como é o caso da PCR, RT-PCR, com suas inúmeras variantes multiplex em tempo real, e os microarrays. Utilizando variantes desses métodos, existem vários kits comerciais diagnósticos para vírus respiratórios. Dessa forma, o diagnóstico etiológico de pneumonias virais é hoje possível em larga escala.
São vários os vírus reconhecidos como causadores de pneumonias. Podem ser subdivididos naqueles que causam doença em indivíduos imunocompetentes, objeto deste artigo de revisão, e nos que atuam de forma oportunista, na vigência de imunodeficiência. Também, as etiologias de pneumonias virais entre imunocompetentes dependem da época do ano, já que várias dessas viroses são sazonais, com predomínio no inverno, ou mesmo epidêmicas, e também dependem do uso de abordagens diagnósticas sensíveis e confiáveis para a detecção viral, sendo que, aqui, entram os métodos moleculares de detecção genômica. São tradicionalmente conhecidos como causadores de pneumonia os vírus influenza A e B; parainfluenza 1, 2 e 3; RSV; e adenovírus.(1)
Não são numerosos os estudos sobre viroses causando pneumonias comunitárias. Entretanto, nos últimos anos, surgem trabalhos sobre esse assunto utilizando múltiplas abordagens diagnósticas e incluindo as modernas técnicas já citadas. Algumas dessas publicações encontram-se resumidas na Tabela 1. Em um trabalho realizado na Nova Zelândia com 300 indivíduos adultos apresentando pneumonia comunitária, 28% eram de etiologia viral e, nesses pacientes, encontraram-se rinovírus (10%), influenza A (8%), influenza B (2%), RSV (4%), adenovírus (4%), coronavírus (2%) e parainfluenza (1%) sendo que, em alguns pacientes, encontrou-se mais de um tipo de vírus. Os autores observaram que os pacientes com pneumonia viral apresentavam mais mialgias do que aqueles com infecção bacteriana. Observaram, também, que a infecção simultânea de rinovírus com pneumococo estava associada a pneumonias mais graves.(9) Em um estudo espanhol com 338 indivíduos adultos apresentando pneumonia comunitária, demonstrou-se que 18% apresentavam vírus detectável e que os agentes encontrados eram influenza A em 44%, parainfluenza em 18%, influenza B em 16%, RSV em 8,2% e adenovírus em 8,2%. Esses autores observaram que, em 66% dos casos de pneumonia viral, os exames radiológicos mostravam infiltrados alveolares, que queixas de tosse com expectoração eram menos frequentes nos casos virais e, ainda, que esses pacientes, em sua maioria, apresentavam-se taquipneicos, com mais de 30 ciclos/min.(10) Pneumonias comunitárias em outras faixas etárias também têm sido estudadas quanto à etiologia viral. Em um estudo grego com 75 crianças em idade escolar apresentando pneumonia comunitária, rinovírus foram observados em 45% dos pacientes, adenovírus em 12%, parainfluenza em 8%, influenza em 7%, RSV em 3% e metapneumovírus em 1%.
Infecções mistas vírus-bactéria foram encontradas em 35% dos casos.(11) Quanto a indivíduos acima de 50 anos com pneumonia comunitária, em um estudo holandês com 107 pacientes, utilizando-se a RT-PCR e a PCR em tempo real como métodos de diagnóstico rápido, detectou-se a presença de vírus influenza em 13% dos pacientes, coronavírus em 5%, rinovírus em 3%, parainfluenza em 1%, RSV em 1% e adenovírus em 0,5%.(12) Autores norte-americanos citaram o vírus da influenza A e o RSV como os mais comumente identificados em pacientes idosos com pneumonia viral.(13)

Portanto, entre os vírus causadores de pneumonia comunitária, encontram-se influenza A e B, RSV, parainfluenza, metapneumovírus, coronavírus, rinovírus, hantavírus e adenovírus. Um esquema da estrutura desses vírus é mostrado na Figura 1.(14)

Os vírus influenza, pertencentes à família Orthomyxoviridae, são envelopados, possuem 8 segmentos de RNA de fita simples e são classificados nos tipos A, B e C. As duas glicoproteínas do envelope viral são alvo da resposta imune do hospedeiro com a produção de anticorpos neutralizantes. A infecção viral inicia-se pela ação da hemaglutinina viral que se liga, na membrana celular, a resíduos de ácido siálico e, ao mesmo tempo, a neuraminidase, outra proteína viral, cliva o ácido siálico, permitindo a liberação e o espalhamento de vírus recém-produzidos pela célula infectada. Conhecem-se pelo menos 16 hemaglutininas antigenicamente específicas (H1-H16) e 9 neuraminidases (N1-N9). Vírus influenza A possuindo H1-H3 são os causadores da maioria dos casos humanos. As outras hemaglutininas são associadas a vírus de aves aquáticas ou de outros mamíferos. As variações antigênicas de H e N contribuem para a natureza epidêmica dos vírus influenza por dois mecanismos: variações antigênicas menores (drift) que alteram epitopos alvo de anticorpos neutralizantes e variações maiores (shift) que ocorrem por embaralhamento de segmentos genômicos virais quando há infecção simultânea por dois tipos de vírus influenza, resultando no nascimento de um mutante. Esse foi o caso dos vírus causadores de pandemias em 1918 (H1N1), 1957 (H2N2) e 1968 (H3N2). Epidemias por vírus influenza A costumam ocorrer anualmente, durante o inverno, perdurando por 6 a 8 semanas no local e produzindo casos de gravidade variável. A transmissão viral dá-se por pequenas partículas aerossolizadas pela tosse ou espirro de indivíduos infectados, e a doença surge após um período de incubação de 2-3 dias. Esse eficiente mecanismo de transmissão, associado a um curto período de incubação, propicia as grandes e explosivas epidemias dessa virose.(15) A Influenza aviária H5N1 surgiu, na última década, como uma ameaça de causar grave pandemia. O vírus, originário da Ásia, se espalhou em aves aquáticas migratórias e causou centenas de infecções humanas com pneumonias graves e altamente letais em diferentes regiões do mundo. Entretanto, até o momento, o vírus H5N1 aviário não se mostrou adaptado à transmissão respiratória inter-humana.(16)
Os RSV, pertencentes à família Paramyxoviridae, são vírus envelopados e com RNA de fita simples não segmentado. Descrevem-se RSV de dois grupos antigênicos, A e B, ambos podendo causar surtos. Os RSV estão associados a quadros de bronquiolite na infância. O genoma dos RSV é mais estável e sem grande atividade mutagênica, quando comparado ao dos vírus influenza. Também, infecções por RSV induzem uma imunidade incompleta, e a reinfecção por esses vírus costuma ocorrer causando doença respiratória de pequena gravidade. O surgimento de pneumonias e de quadros de maior gravidade aumenta com a idade. Entre idosos internados em asilos, estima-se que 10% desenvolvam RSV anualmente e, desses, 10% desenvolvam pneumonia.(17)
Os vírus parainfluenza, como os RSV, são Paramyxoviridae e estão associados à bronquite e à pneumonia em lactentes. São reconhecidos três tipos virais, numerados de 1 a 3, causando 4-14% das infecções respiratórias. Esses vírus produzem infecções recorrentes, na idade adulta inclusive, com pneumonia em jovens e broncopneumonia em idosos.(18)
Os metapneumovírus humanos, como os parainfluenza e os RSV, são Paramyxoviridae. Foram descritos recentemente em 2001. São de distribuição mundial, tendo sido observados a causar bronquiolite e pneumonia em lactentes.(19) Em adultos jovens, produzem resfriados, quadros gripais e exacerbação de crises asmáticas. Infecções respiratórias por metapneumovírus também ocorrem em idosos com doenças cardiopulmonares.(13)
Os coronavírus, da família Coronaviridae, são comumente associados a resfriados e mostram-se de difícil isolamento em laboratório. As cepas OC43 e 229E são as mais comuns, tendo sido descritas em todas as faixas etárias. Em 2002, surgiu na China um novo coronavírus, causando uma nova doença, que foi denominada SARS. Essa doença se espalhou rapidamente pelo mundo em viajantes oriundos da China, causando pneumonias que cursavam com insuficiência respiratória e alta letalidade, chegando a representar uma grave ameaça à saúde pública mundial. Entretanto, provavelmente, por não ser um vírus de fácil transmissão, a epidemia arrefeceu em alguns meses.(20)
Os rinovírus, da família Picornaviridae, são os causadores mais comuns dos resfriados, que ocorrem entre pessoas de todas as idades e durante todo o ano. O papel dos rinovírus como causadores primários de pneumonia é controverso. Entretanto, o vírus tem sido recuperado das vias aéreas baixas de recém-nascidos e de imunodeprimidos com pneumonia.(21)
Os hantavírus pertencem à família Bunyaviridae. A infecção humana por esses vírus leva à SPCVH, uma doença emergente nas Américas, causada pela aspiração de aerossóis dos dejetos de roedores silvestres (Sigmodontinae) contaminados por esses vírus. No Brasil, ocorre SPCVH desde 1993, com cerca de 1.100 casos notificados até 2008. São conhecidos no Brasil como causadores de doença humana os hantavírus Araraquara, Juquitiba, Castelo dos Sonhos, Rio Mearim e Laguna Negra-símile. Analisando, entre 1998 e 2007, uma casuística de 70 pacientes com SPCVH na região de Ribeirão Preto (SP), observou-se uma maior incidência da doença entre abril e setembro, época de estiagem. Dentre os pacientes, 75,7% eram do sexo masculino, com 35,8 ± 11,7 anos em média. Esses pacientes, após incubação de 2 a 30 dias, apresentaram dispneia (87%), febre (81%), tosse (44%) e cefaleia (34%), por 3-6 dias (média de 4), sintomas acompanhados de taquicardia (81%), hipotensão arterial (56%), diminuição da SaO2 (49%), acidose metabólica (57%), linfocitopenia (51%), hematócrito > 45 mg% (70%), leucocitose com desvio à esquerda (67%), creatinina (51%) e ureia (42%) séricas elevadas. Além disso, apresentavam alteração radiológica com velamento bilateral, difuso, intersticial entre o primeiro e o quarto dia de doença e seguindo com alveolização, como mostra a Figura 2. A evolução para insuficiência respiratória, hipotensão arterial e choque ocorreu 24-48 h após o início dos sintomas, e a maior frequência de óbitos ocorreu no quarto dia de doença. Hematócrito elevado e plaquetopenia foram sinais fortemente sugestivos da doença. Também, a hipótese diagnóstica de pneumonia atípica foi associada a bom prognóstico (p = 0,0136) e, por outro lado, a infusão hídrica parenteral de mais que 2.000 mL e a hipotensão arterial foram associadas a mau prognóstico (p = 0,0286 e p = 0,0453, respectivamente). O contato com roedores infectados mostrou-se um fator de risco para a transmissão e a morbidade (p = 0,0198). Nessa casuística de indivíduos com SPCVH causada pelo vírus Araraquara, a letalidade foi de 54,3%.(22)

Os adenovírus, vírus de DNA pertencentes à família Adenoviridae, ocorrem em todo o mundo. Adenovírus de 52 sorotipos podem ser a causa de infecções assintomáticas, faringites, ceratoconjuntivites, gastroenterite, cistite hemorrágica, meningoencefalite, hepatite, miocardite e doença disseminada grave. Pneumonias graves causadas pelo sorotipo 14 foram descritas em adultos e crianças com DPOC leve e moderada.(23)
O tratamento das pneumonias por vírus depende da gravidade do quadro e do agente infectante. Medidas gerais de suporte, especialmente aquelas com ventilação, para tratamento da hipóxia, podem ser críticas para a sobrevida do paciente. A alta frequência com que infecções bacterianas se associam às virais faz com que antibióticos, após a análise microbiológica, possam estar indicados nesses casos. A terapia antiviral está indicada nos casos graves e em imunocomprometidos, baseada nos testes de diagnóstico para vírus. Essa terapia costuma mostrar-se mais eficaz quando iniciada precocemente, ao surgimento da sintomatologia.(3)
Quatro drogas antivirais podem ser utilizadas no tratamento das infecções por vírus influenza (amantadina, rimantadina, zanamivir e oseltamivir). Amantadina e rimantadina são ativas contra vírus influenza A. Zanamivir e oseltamivir são ativos contra influenza A e B. Essas drogas reduzem a gravidade da doença e a sintomatologia quando iniciado o seu uso nas primeiras 48 h de doença. Zanamivir e oseltamivir, em pacientes com doença pulmonar obstrutiva, podem induzir broncoespasmo.
Entretanto, apesar de não ter sua eficiência definitivamente comprovada por estudos científicos, é indicado o tratamento antiviral de pacientes com pneumonia por influenza.(13)
Para evitar a introdução de vírus respiratórios epidêmicos e causadores de doença grave, como é o caso da influenza (H5N1) aviária, recomenda-se o isolamento respiratório imediato do paciente em enfermaria, com pressão negativa e filtração do ar. Um mínimo de profissionais deve lidar com o paciente, sempre usando máscara N95 e atentos à lavagem das mãos. Além disso, todos os objetos usados pelo paciente devem ser descontaminados.(24)
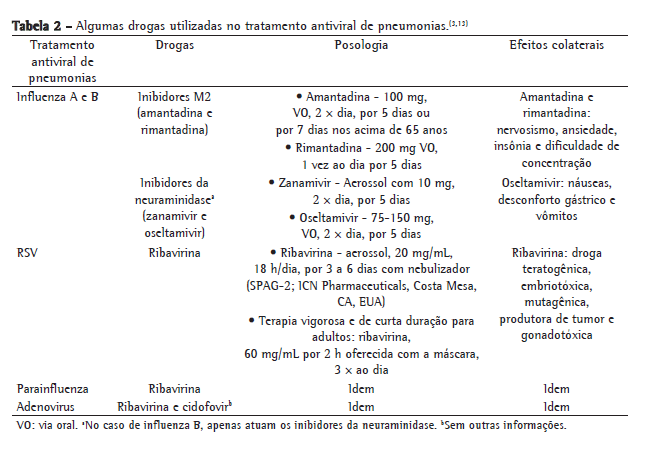
O tratamento da pneumonia por RSV inclui medidas de suporte ventilatório e o uso do aerossol de ribavirina, como mostra a Tabela 2.
Nas pneumonias por hantavírus, que fazem parte do quadro da SPCVH, a suspeita clínica precoce é muito importante, pois tem impacto na sobrevida dos pacientes. Essa suspeita tem por base os aspectos clínicos, laboratoriais e radiológicos. A suspeita clínica precoce permite uma rápida instituição de medidas de suporte cardiorrespiratório, visto que, nesse caso, ainda não há tratamento antiviral específico.
Recomenda-se que o paciente seja rapidamente transferido para uma unidade de terapia intensiva. O ajuste hemodinâmico está indicado nos casos mais graves, utilizando a medida de pressão da artéria pulmonar (Swan-Ganz) e uma reposição volêmica criteriosa. A análise de volume infundido em 10 pacientes com SPCVH mostrou uma associação significante entre volumes superiores a 2.500 mL nas primeiras 24 h de tratamento hospitalar e a evolução para o óbito. O volume infundido tende a se concentrar nos pulmões, agravando a insuficiência respiratória. Desse modo, preconiza-se, para a estabilização hemodinâmica, a utilização de drogas vasoativas (dobutamina, associada ou não à noradrenalina). O uso precoce dessas aminas é útil para prevenir o choque e a depressão miocárdica que acompanham as formas graves dessa doença. O uso de corticosteroide é controverso nesses casos.
Entretanto, tomando como base o mecanismo fisiopatológico da doença, esse uso seria benéfico por reduzir a intensa atividade de mediadores inflamatórios. O suporte ventilatório deve dar preferência a estratégias de proteção pulmonar, evitando-se altas frações inspiradas de oxigênio e limitando-se a pressão de vias aéreas para evitar volutrauma e barotrauma. A droga ribavirina mostra-se eficaz in vitro contra hantavírus; porém, sua atividade in vivo carece de comprovação cientifica.(25)
Quanto ao tratamento da SARS, além das medidas de suporte ventilatório, recomenda-se, embora sem comprovação da eficácia em estudo controlado, o uso da ribavirina ou até de uma combinação incluindo ribavirina com lopinavir/ritonavir e corticosteroide, preconizada com base na redução da carga viral como a terapia mais eficaz.
A prevenção das infecções virais que causam pneumonia comunitária inclui, dependendo do vírus, vacinas e imunização passiva. Para a prevenção das infecções por Influenza A, existem vacinas de vírus inativado produzidas em ovos embrionados e que possuem um painel desses vírus com circulação na região.(26) Para prevenir as infecções por RSV, particularmente em crianças de alto risco, utilizam-se as seguintes imunoglobulinas: RSV-IGIV (RespiGamT; Massachusetts Public Health Biologic Laboratories, Boston, MA, EUA) e palivizumab (Synagis®, Abbott, São Paulo, Brasil).(3)
Referências 1. Nolte FS. Molecular diagnostics for detection of bacterial and viral pathogens in community-acquired pneumonia. Clin Infect Dis. 2008;47 Suppl 3:S123-6.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-25.
3. Treanor JJ. Respiratory infections. In: Richman DD, Whitley RJ, Hayden FG. Clinical Virology. Washington: ASM Press; 2002. p.7-26.
4. Ng WF, To KF, Lam WW, Ng TK, Lee KC. The comparative pathology of severe acute respiratory syndrome and avian influenza A subtype H5N1--a review. Hum Pathol. 2006;37(4):381-90.
5. Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12(1):48-54.
6. Borges AA, Figueiredo LT. Atualização de conhecimentos sobre a patogênese da síndrome pulmonar e cardiovascular por hantavírus. Rev Patol Trop. 2007;36(3):191-204.
7. Buchholz UJ, Ward JM, Lamirande EW, Heinze B, Krempl CD, Collins PL. Deletion of nonstructural proteins NS1 and NS2 from pneumonia virus of mice attenuates viral replication and reduces pulmonary cytokine expression and disease. J Virol. 2009;83(4):1969-80.
8. Moreno L, Krishnan JA, Duran P, Ferrero F. Development and validation of a clinical prediction rule to distinguish bacterial from viral pneumonia in children. Pediatr Pulmonol. 2006;41(4):331-7. Erratum in: Pediatr Pulmonol. 2006;41(5):494.
9. Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63(1):42-8.
10. de Roux A, Marcos MA, Garcia E, Mensa J, Ewig S, Lode H, et al. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125(4):1343-51.
11. Tsolia MN, Psarras S, Bossios A, Audi H, Paldanius M, Gourgiotis D, et al. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004;39(5):681-6.
12. Oosterheert JJ, van Loon AM, Schuurman R, Hoepelman AI, Hak E, Thijsen S, et al. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41(10):1438-44.
13. Falsey AR, Walsh EE. Viral pneumonia in older adults. Clin Infect Dis. 2006;42(4):518-24.
14. Flores EF. Estrutura das partículas víricas. In: Flores EF, organizer. Virologia Veterinária. Santa Maria: UFSM; 2007. p.21-9.
15. Centers for Disease Control and Prevention (CDC). Pneumonia and influenza death rates--United States, 1979-1994. MMWR Morb Mortal Wkly Rep. 1995;44(28):535-7. Erratum in: MMWR Morb Mortal Wkly Rep 1995;44(41):782.
16. Enserink M. Avian influenza. Infection study worries farmers, bird lovers. Science. 2009;323(5912):324.
17. Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179-86.
18. Parainfluenza infections in the elderly 1976-82. Br Med J (Clin Res Ed). 1983;287(6405):1619.
19. van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719-24.
20. McIntosh K, Anderson LJ. Coronaviruses. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practices of infectious diseases. Philadelphia: Churchill Livingston; 2005. p. 1990-8.
21. Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181(6):1875-84.
22. Campos GM. Síndrome Pulmonar e Cardiovascular por Hantavirus: estudos sobre uma doença emergente [thesis]. Ribeirão Preto: Universidade de São Paulo; 2008.
23. Louie JK, Kajon AE, Holodniy M, Guardia-LaBar L, Lee B, Petru AM, et al. Severe pneumonia due to adenovirus serotype 14: a new respiratory threat? Clin Infect Dis. 2008;46(3):421-5.
24. Bell DM; World Health Organization Writing Group. Non-pharmaceutical interventions for pandemic influenza, national and community measures. Emerg Infect Dis. 2006;12(1):88-94.
25. Figueiredo LT. Hantavirose. In: Cimerman S, Cimerman B, editors. Condutas em infectologia. São Paulo: Atheneu; 2004. p.123-32.
26. Luke CJ, Subbarao K. Vaccines for pandemic influenza. Emerg Infect Dis. 2006;12(1):66-72.
Trabalho realizado na Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto (SP) Brasil.
Endereço para correspondência: Luiz Tadeu Moraes Figueiredo. Centro de Pesquisa em Virologia, Faculdade de Medicina de Ribeirão Preto - USP, Av. Bandeirantes, 3900, CEP 14049-900, Ribeirão Preto, SP, Brasil.
Tel 55 16 3602-3271. E-mail: ltmfigue@fmrp.usp.br
Apoio financeiro: Nenhum.
Recebido para publicação em 26/1/2009. Aprovado, após revisão, em 26/3/2009.
Sobre os autoresLuiz Tadeu Moraes Figueiredo
Professor Titular. Disciplina de Doenças Infecciosas e Tropicais, Departamento de Clínica Médica, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto (SP) Brasil.





