ABSTRACT
Some chronic respiratory diseases can cause hypoxemia and, in such cases, long-term home oxygen therapy (LTOT) is indicated as a treatment option primarily to improve patient quality of life and life expectancy. Home oxygen has been used for more than 70 years, and support for LTOT is based on two studies from the 1980s that demonstrated that oxygen use improves survival in patients with COPD. There is evidence that LTOT has other beneficial effects such as improved cognitive function, improved exercise capacity, and reduced hospitalizations. LTOT is indicated in other respiratory diseases that cause hypoxemia, on the basis of the same criteria as those used for COPD. There has been an increase in the use of LTOT, probably because of increased life expectancy and a higher prevalence of chronic respiratory diseases, as well as greater availability of LTOT in the health care system. The first Brazilian Thoracic Association consensus statement on LTOT was published in 2000. Twenty-two years la-ter, we present this updated version. This document is a nonsystematic review of the literature, conducted by pulmonologists who evaluated scientific evidence and international guidelines on LTOT in the various diseases that cause hypoxemia and in specific situations (i.e., exercise, sleep, and air travel). These recommendations, produced with a view to clinical practice, contain several charts with information on indications for LTOT, oxygen sources, accessories, strategies for improved efficiency and effectiveness, and recommendations for the safe use of LTOT, as well as a LTOT prescribing model.
Keywords:
Oxygen; Hypoxia; Oxygen inhalation therapy; Delivery of health care.
RESUMO
Algumas doenças respiratórias crônicas podem evoluir com hipoxemia e, nessas situações, a oxigenoterapia domiciliar prolongada (ODP) está indicada como opção terapêutica com o objetivo principal de melhorar a qualidade e a expectativa de vida desses pacientes. O oxigênio domiciliar é usado há mais de 70 anos, e a ODP tem como base dois estudos da década de oitenta que demonstraram que o uso de oxigênio melhora a sobrevida de pacientes com DPOC. Existem evidências de que a ODP tem outros efeitos benéficos como melhora da função cognitiva e da capacidade de exercício e redução de hospitalizações. A ODP está indicada para outras doenças respiratórias que cursam com hipoxemia, segundo os mesmos critérios estabelecidos para a DPOC. Tem sido observado aumento no uso da ODP provavelmente pela maior expectativa de vida, maior prevalência de doenças respiratórias crônicas e maior disponibilidade de ODP no sistema de saúde. O primeiro consenso sobre ODP da Sociedade Brasileira de Pneumologia e Tisiologia foi publicado em 2000; após 22 anos, apresentamos esta versão atualizada. Este documento é uma revisão não sistemática da literatura, realizada por pneumologistas que avaliaram evidências científicas e diretrizes internacionais sobre ODP nas diversas doenças que cursam com hipoxemia e em situações específicas (exercício, sono e viagens aéreas). Estas recomendações, tendo em vista a prática clínica, oferecem diversos quadros com informações sobre indicações, fontes de oxigênio, acessórios e estratégias para melhor eficiência, efetividade e uso seguro da ODP, assim como um modelo para sua prescrição.
Palavras-chave:
Oxigênio; Hipóxia; Oxigenoterapia; Atenção à saúde.
INTRODUÇÃO As doenças respiratórias crônicas podem evoluir com hipoxemia em repouso ou induzida pelo exercício, estando entre as principais causas de redução da qualidade e da expectativa de vida. Elas têm como consequência, especialmente devido às complicações infecciosas e internações, um alto custo para a assistência à saúde pública e suplementar, assim como para os pacientes e seus familiares. Para aqueles que progridem com hipoxemia, a prescrição da oxigenoterapia domiciliar prolongada (ODP) poderá trazer benefícios, como redução da sensação de dispneia, maior tolerância aos esforços e melhora na expectativa de vida.
O oxigênio domiciliar é usado empiricamente há mais de 70 anos, e o uso da ODP tem como base dois estudos de referência da década de 80: o do Nocturnal Oxygen Therapy Trial Group(1) e o do Medical Research Council Working Party.(2) Ambos demonstraram que o uso de oxigênio melhorava a sobrevida dos pacientes com DPOC.
A melhora da sobrevida com a ODP foi comprovada em pacientes com DPOC estável e hipoxemia crônica grave.(3-6) Nas últimas décadas, acumularam-se evidências de que a ODP tem outros efeitos benéficos, tais como redução da depressão, melhora da função cognitiva, melhora da qualidade de vida, melhora da capacidade de exercício e redução de hospitalizações.(7-16) Além disso, a ODP pode estabilizar ou mesmo reverter a hipertensão pulmonar (HP) e diminuir as arritmias cardíacas e a isquemia miocárdica em pacientes com DPOC.(17,18) Entretanto, o uso de ODP para outras doenças respiratórias que cursam com hipoxemia grave é baseado em uma extrapolação de dados relativos à DPOC, respaldados amplamente nos conhecimentos da fisiologia respiratória e respiração celular, que são idênticas independentemente da doença que causou a hipoxemia, assim como suas repercussões sistêmicas.
O primeiro consenso sobre ODP da Sociedade Brasileira de Pneumologia e Tisiologia foi publicado em 2000 e continua como referência para muitos protocolos de oxigenoterapia em nosso meio.(19) Nesses últimos 22 anos, houve grande aumento no uso da ODP, em parte devido ao aumento da expectativa de vida e ao número crescente de pacientes diagnosticados com doenças pulmonares crônicas, com destaque para a DPOC e as doenças pulmonares intersticiais (DPI), além do fato de que a ODP está mais disponível no sistema de saúde. Além disso, nos últimos 2 anos, uma parcela de sobreviventes da COVID-19 precisou utilizar oxigênio por um período de transição ou se tornaram usuários crônicos.
No Brasil, os protocolos de fornecimento gratuito da ODP são municipais ou estaduais, e esse é um dos motivos para não dispormos de dados nacionais confiáveis sobre o número total de usuários. Nos EUA, mais de 1,5 milhão de pacientes faziam uso de ODP em 2018.(20)
Em nosso meio, o acesso à ODP é garantido pelas Leis Orgânicas do Sistema Único de Saúde (Leis Federais n. 8080/90 e n. 8142/90) que dispõem sobre as condições para a promoção, proteção e recuperação da saúde e a garantia desse direito a todo cidadão.
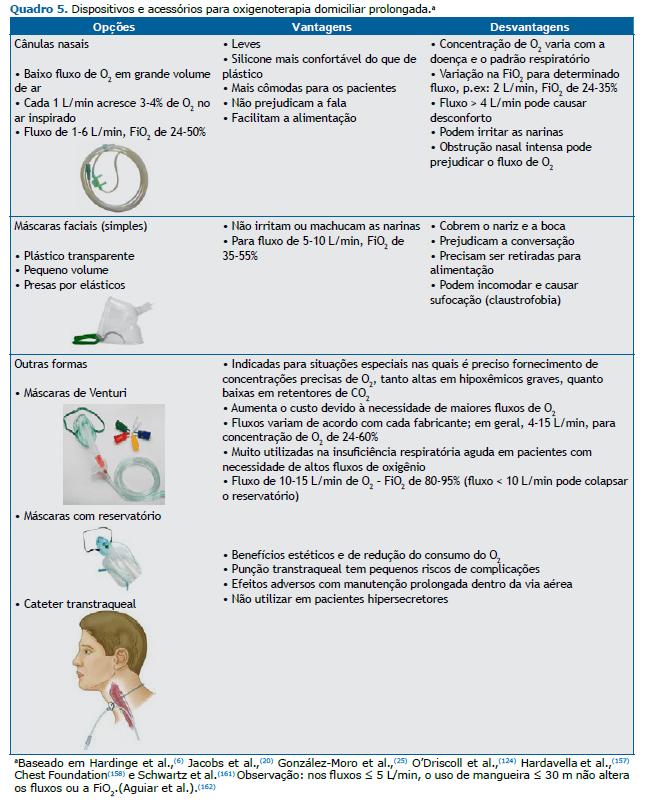
Foram reunidos 16 pneumologistas com expertise em oxigenoterapia, os quais revisaram de forma não sistemática a literatura e diretrizes internacionais em busca de evidências científicas sobre ODP nas diversas doenças que cursam com hipoxemia e sobre o uso de oxigênio em situações específicas (sono, exercício e viagens aéreas). Com ênfase na prática clínica elaboramos diversos quadros para facilitar o manejo dos pacientes com as principais indicações, fontes diversas para fornecimento de oxigênio e acessórios necessários, estratégias para melhor adesão, eficiência, efetividade, redução de custos e uso seguro da ODP, bem como um modelo para sua prescrição (Quadros 1-10 e Figura 1). Nestas recomendações, optamos pelo termo ODP apesar de entendermos que o sentido é mais amplo, ou seja, é suplementação de oxigênio para pacientes não hospitalizados que estejam em tratamento ambulatorial, para uso durante qualquer atividade, dentro ou fora do seu domicílio.


O oxigênio é fundamental na fosforilação oxidativa, promovendo a síntese de ATP para a produção de energia. O conteúdo arterial de oxigênio depende da pressão parcial de oxigênio inspirado, que, por sua vez, depende da pressão atmosférica, da ventilação, da troca gasosa realizada e da concentração de hemoglobina e de sua afinidade pelo oxigênio.(21)
Apenas uma pequena fração desse conteúdo (menos de 2%) encontra-se dissolvida no plasma, livre da hemoglobina. Ao nível do mar, a SpO2 de 96-98% corresponde a aproximadamente 20 mL de oxigênio a cada 100 mL de sangue.(22) A oferta de oxigênio aos tecidos, por sua vez, depende do conteúdo arterial de oxigênio e do débito cardíaco (DC). Quanto menor a concentração de oxigênio, maior a afinidade da hemoglobina pelo oxigênio e, consequentemente, menor a sua oferta aos tecidos. O aumento da temperatura corporal, a acidose por qualquer causa e/ou o aumento do 2,3-difosfoglicerato deslocam a curva de dissociação da hemoglobina para a direita, diminuindo a afinidade ao oxigênio e aumentando sua oferta para os tecidos.(23)
A avaliação da hipoxemia pode ser feita através do cálculo da diferença alvéolo-arterial de oxigênio (P(A-a)O2) ou do cálculo do índice de oxigenação (PaO2/FiO2). Em pacientes hipoxêmicos com P(A-a)O2 dentro da normalidade o provável mecanismo fisiopatológico é a presença de hipoventilação. Naqueles com aumento da diferença e sem correção da hipoxemia com suplementação de oxigênio e/ou com aumento da FiO2, sugere-se a presença de shunt cardíaco ou intrapulmonar. Já naqueles com resposta, deve-se considerar desequilíbrio na ventilação-perfusão (V/Q) ou alteração da difusão.(24)
A hipoxemia aguda ou crônica induz várias respostas fisiológicas com o objetivo de manter o fornecimento adequado de oxigênio aos tecidos. Quando a PaO2 encontra-se abaixo de 60 mmHg, ocorre aumento do estímulo ventilatório, elevando a PaO2 e reduzindo a PaCO2. Os leitos vasculares que irrigam o tecido hipóxico se dilatam, induzindo a taquicardia compensatória para aumento do DC e melhora da oferta de oxigênio. A vasculatura pulmonar se contrai com o intuito de melhorar a relação V/Q nas áreas comprometidas. Se não houver resolução da hipoxemia, ocorrerá ativação renal para aumentar a produção de eritropoetina e estimular a eritrocitose, elevando a capacidade de transporte e fornecimento de oxigênio. Esses benefícios iniciais podem ter efeitos prejudiciais em longo prazo, uma vez que a vasoconstrição prolongada, a eritrocitose e o aumento do DC podem causar HP e insuficiência ventricular direita, diminuindo a sobrevida. Além disso, o custo energético decorrente do aumento da ventilação e da demanda de oxigênio pode contribuir para a desnutrição em pacientes com DPOC.(21-24)
DEFINIÇÃO DE HIPOXEMIA Ao nível do mar, a pressão barométrica é de 760 mmHg (ou 1 atm), a FiO2 é de 0,21 (ou denominada pela prática clínica de 21%, terminologia que é usada nos trabalhos científicos e que será utilizada neste documento) e a PaO2 é de 80-100 mmHg em indivíduos saudáveis. Portanto, a PaO2 depende da altitude, mas também deve ser corrigida para a idade (com o envelhecimento temos uma redução progressiva da PaO2). A hipoxemia é definida quando a PaO2 encontra-se abaixo do limite inferior da normalidade; entretanto, isso não significa obrigatoriamente que haverá necessidade de suplementação de oxigênio.
Classicamente, a indicação de ODP é baseada em trabalhos sobre DPOC e extrapolada para as demais doenças respiratórias crônicas. A oximetria de pulso é usada para triar os pacientes com hipoxemia em repouso; quando a SpO2 for ≤ 92%, está indicada a solicitação de gasometria arterial coletada em ar ambiente; além disso, também se deve avaliar a presença ou não de hipercapnia. Os critérios para indicação de ODP são os seguintes: hipoxemia grave com PaO2 ≤ 55 mmHg ou SaO2 ≤ 88% ou PaO2 ≤ 59 mmHg ou SaO2 ≤ 89% na presença de edema, cor pulmonale (HP) e/ou policitemia (hematócrito > 55%).(6,20,25) A duração diária da ODP deve ser de, no mínimo, 15 h/dia, incluindo o período de sono, e o fluxo de oxigênio deve ser o suficiente para elevar a PaO2 acima de 60 mmHg ou a SpO2 acima de 90%.(6,20,25)
Para hipoxemia induzida pelo exercício, utiliza-se como definição a redução ≥ quatro pontos da SpO2 durante o esforço, mesmo que a avaliação basal da SpO2 esteja dentro dos valores de normalidade. A ODP nessa situação é controversa na literatura, devendo-se considerar a melhora da sensação da dispneia após a suplementação de oxigênio ou para uso durante a reabilitação pulmonar.
Os mecanismos de ação da suplementação de oxigênio vão além da correção da hipoxemia e da melhor oferta de oxigênio para as células. Em indivíduos normais expostos a condições hipoxêmicas, o acúmulo de fatores induzidos pela hipoxemia no núcleo das células regula positivamente vários genes responsáveis pelas respostas fisiológicas à hipóxia, incluindo o remodelamento da vasculatura pulmonar, culminando em HP e aumento da eritropoiese.(26,27) Estudos limitados, baseados em modelos animais, sugerem que alguns dos efeitos terapêuticos da ODP são mediados pela inibição dos fatores induzidos pela hipoxemia.(28)
ODP EM PACIENTES COM DPOC Embora estudos observacionais tenham sugerido benefícios da utilização de oxigênio suplementar na DPOC, dois ensaios clínicos randomizados (ECR) foram fundamentais para estabelecer as bases para o uso da ODP.(1,2) O estudo do Medical Research Council Working Party(2) acompanhou por 5 anos 87 pacientes com DPOC que apresentavam hipoxemia grave, hipercapnia e cor pulmonale. Os pacientes foram randomizados em dois grupos, grupo placebo (sem suplementação de oxigênio) e grupo tratamento com ODP por no mínimo 15 h/dia. No término do seguimento, a mortalidade foi 45,2% no grupo que recebeu oxigênio e 66,7% no grupo controle.(2) O ECR do Nocturnal Oxygen Therapy Trial Group(1) randomizou em dois grupos 203 pacientes hipoxêmicos com DPOC: suplementação contínua de oxigênio ou somente durante a noite por 12 h. O tempo de seguimento foi de 12 meses, e a mortalidade foi maior no grupo que recebeu oxigênio noturno (razão de risco = 1,94; p = 0,01).
Os estudos que demonstraram aumento de sobrevida com a ODP incluíram pacientes com hipoxemia grave (PaO2 ≤ 55 mmHg ou SaO2 ≤ 88%). Por outro lado, o uso da ODP em portadores de DPOC com hipoxemia moderada não mostrou benefícios na sobrevida.(29-31) Além desses estudos, outros realizados em indivíduos com DPOC demonstram que a ODP tem outros benefícios em pacientes hipoxêmicos graves com DPOC, tais como melhora da qualidade de vida, da capacidade de exercício e da capacidade cognitiva, assim como redução da resistência vascular pulmonar, da pressão no átrio direito, da morbidade cardiovascular e da frequência de hospitalizações.(13,15-18,32-34)
Como mencionado anteriormente, os mecanismos de ação da suplementação de oxigênio vão além da correção da hipoxemia e da melhor oferta de oxigênio para as células. Em indivíduos normais expostos a condições hipoxêmicas, o acúmulo de fatores induzidos pela hipoxemia no núcleo das células regula positivamente vários genes responsáveis pelas respostas fisiológicas à hipóxia, incluindo o remodelamento da vasculatura pulmonar, que leva à HP e aumento da eritropoiese. (26,27) Estudos limitados, baseados em modelos animais, sugerem que alguns dos efeitos terapêuticos da ODP são mediados pela inibição dos fatores induzidos pela hipoxemia.(28)
Na DPOC, a ODP é indicada para pacientes com hipoxemia grave persistente, clinicamente estáveis, e com terapia medicamentosa otimizada por, no mínimo, um mês. Aqueles instáveis como, por exemplo, após uma exacerbação recente, devem receber suplementação de oxigênio temporariamente até reavaliação clínica em 1-3 meses após a descompensação, uma vez que cerca de 50% não necessitarão de ODP no seguimento. (35,36) Recomenda-se que todos os pacientes avaliem a necessidade de aumentar o fluxo de oxigênio durante o exercício e o sono. Os fluxos excessivos de oxigênio devem ser evitados para minimizar os efeitos colaterais do oxigênio, especialmente a piora da hipercapnia em pacientes retentores de gás carbônico, com aumento do risco de depressão do sensório e, em casos extremos, de coma por narcose,(37) sugerindo-se manter a SpO2 em 90-92%.
A oximetria de pulso é usada para triar os pacientes com hipoxemia; quando a SpO2 for ≤ 92%, é indicada a realização de gasometria arterial. A gasometria arterial é necessária para indicar a ODP e também é útil para detectar a presença de hipercapnia. Como mencionado anteriormente, os critérios para indicação de ODP são PaO2 ≤ 55 mmHg ou SaO2 ≤ 88% ou PaO2 ≤ 59 mmHg ou SaO2 ≤ 89% na presença de edema, HP, cor pulmonale e/ou policitemia (hematócrito > 55%).(6,20,25)
Os pacientes hipoxêmicos com suspeita de síndrome da apneia obstrutiva do sono (AOS) ou de hipoventilação alveolar devem ser encaminhados para polissonografia. Vale ressaltar que nessas situações a correção da hipoxemia pode ocorrer somente com o uso de ventilação não invasiva, mesmo sem suplementação de oxigênio.(38)
Em relação aos fumantes, esses devem ser encaminhados para programas de cessação do tabagismo e orientados a não fumar durante o uso de oxigênio, não porque seja um gás inflamável, mas por ser um acelerador da combustão e aumentar o risco de incêndios e explosões.(39) Além disso, fumar aumenta a concentração de monóxido de carbono no sangue, que tem alta afinidade com a hemoglobina e reduz o transporte do oxigênio.(6,25,27)
A adesão ao tratamento é fundamental para alcançar os benefícios esperados com a ODP. A adesão pode variar de 45% a 70% e pode ser melhorada através da identificação de barreiras, facilitadores e atitudes dos prescritores.(40) Muitos pacientes usam o oxigênio por menos de 15 h/dia e/ou com fluxos abaixo do prescrito por seus médicos devido à falta de orientações sobre a sua doença e o papel do oxigênio no tratamento, pouca melhora dos seus sintomas ou medo de ficar dependente da ODP.(6,20,25,41,42) A qualidade do suporte da equipe de saúde melhora a adesão do paciente ao uso correto do oxigênio (Quadro 6). Da mesma maneira que a indicação deve ser bem avaliada, a suspensão da ODP deve ser criteriosa. Oba et al.(43) observaram que apenas 35% dos pacientes foram reavaliados corretamente e que a taxa de reavaliação adequada foi significativamente maior entre os pneumologistas do que entre os generalistas (65% vs. 17%).
ODP NAS DEMAIS DOENÇAS PULMONARES CRÔNICAS Fibrose cística Na fibrose cística (FC) a infecção crônica e recorrente das vias aéreas causa danos pulmonares que resultam em hipoxemia crônica, sendo a insuficiência respiratória a principal causa de morte.(44,45) A proporção de pacientes com FC em uso de oxigênio é desconhecido, e o impacto da ODP na sobrevida e na qualidade de vida desses pacientes permanece incerto.(45) Uma revisão(45) incluindo 11 estudos sobre o uso de oxigênio em pacientes com FC, com apenas 1 estudo sobre seu benefício de longo prazo, concluiu que a ODP não teve efeito perceptível sobre a mortalidade, frequência de hospitalização e progressão da doença comparado ao grupo sem uso de oxigênio, apesar da redução do absenteísmo na escola e no trabalho.
Há poucas evidências para a indicação da ODP em pacientes com FC avançada, ainda que em curto prazo tenha sido demonstrada alguma melhora na PaO2 durante o sono e o exercício.(46) Nas diretrizes da Cystic Fibrosis Foundation,(47) é sugerido que pacientes com FC avançada sejam avaliados anualmente quanto a hipoxemia de esforço e noturna, hipercapnia e HP, sendo preconizado o uso de oxigênio em pacientes com SpO2 ≤ 88% durante o sono ou induzida pelo exercício. As diretrizes da British Thoracic Society(6) recomendam que sejam utilizados os mesmos critérios de indicação usados para a DPOC na FC.
DPI A DPOC e as DPI são as principais indicações de ODP. (48) Os grandes e recentes ECR em fibrose pulmonar idiopática concluíram que 21-28% dos participantes dos estudos receberam terapia de oxigênio suplementar. (49,50) No entanto, essas taxas não diferenciaram hipoxemia em repouso ou isolada de esforço. Um estudo retrospectivo com 400 pacientes na Austrália em clínicas especializadas em DPI relatou uma prevalência de hipoxemia em repouso de 3,5%.(51)
As definições de hipoxemia de esforço variam amplamente, mas independentemente disso, ela é comum em pacientes com DPI, inclusive sendo mais grave do que na DPOC quando comparada com a gravidade do comprometimento da função pulmonar. Além disso, a hipoxemia de esforço é um fator de pior prognóstico para esses pacientes.(52-55) A hipoxemia noturna acomete aproximadamente 27% dos pacientes, e a associação com distúrbios respiratórios do sono pode aumentar essa prevalência.(56)
O benefício da ODP nas DPI é incerto. Uma revisão sistemática(57) não mostrou efeitos consistentes sobre a dispneia durante o esforço, embora a capacidade de exercício tenha melhorado. Os estudos sobre uso de ODP em DPI têm alto risco de viés, não sendo possível estimar o seu impacto na sobrevida.(57) As diretrizes clínicas atuais têm consistentemente recomendado a ODP para pacientes com DPI com os mesmos critérios utilizados para pacientes com DPOC.(4,6,58-62)
HP A HP pré-capilar é um diagnóstico hemodinâmico que engloba hipertensão arterial pulmonar (HAP, grupo I), HP por doenças respiratórias (grupo III) e HP tromboembólica crônica (grupo IV), e pode evoluir com hipoxemia.(63) Há vários mecanismos fisiopatológicos envolvidos na hipoxemia, como a redução do DC dos pacientes com HP, alteração da relação V/Q, shunt direita-esquerda e redução da capacidade de difusão do oxigênio, podendo ser intensificada pela vasoconstrição pulmonar.(64-66)
Um estudo realizado por Ulrich et al.(64) demonstrou que o uso de oxigênio suplementar na HAP e na HP tromboembólica crônica resultou em benefício na capacidade de exercício e na qualidade de vida. Além disso, houve melhora na SpO2 noturna e nos transtornos do sono naqueles com hipoxemia induzida pelo exercício e distúrbios do sono (apneias do sono e hipoxemia noturna). Embora o tempo de suplementação de oxigênio tenha sido curto (até 5 semanas), não se sabe se os efeitos positivos da suplementação de oxigênio durante o exercício se refletirão em benefícios em longo prazo.(67,68) Um estudo observacional sobre HAP concluiu que o risco de morte foi significativamente maior entre os pacientes com DLCO acentuadamente reduzida (< 40% do predito) sem suplementação de oxigênio quando comparados com os que receberam a suplementação. No entanto, esse último grupo foi medicado com mais fármacos específicos para HAP, caracterizando um viés de seleção.(69)
O uso de ODP em pacientes adultos com síndrome de Eisenmenger permanece controverso, com poucos dados na literatura. Um estudo prospectivo controlado(70) não mostrou impacto do uso de oxigênio noturno sobre capacidade de exercício, história natural da doença e sobrevida no período de seguimento de 2 anos. Assim, o uso da ODP é facultativo nesses pacientes, e a indicação deverá ser individualizada.(70)
As recomendações das diretrizes sobre o uso de oxigênio suplementar na HP são controversas provavelmente devido à ausência de estudos de longo prazo.(71) Apesar de evidências limitadas, sugere-se que a ODP seja prescrita para pacientes com HP e PaO2 < 60 mmHg, considerando o benefício sintomático e a correção da dessaturação aos esforços.(6,25)
ODP NAS DOENÇAS CRÔNICAS AVANÇADAS E CUIDADOS PALIATIVOS Promover intervenções precoces que não apenas aliviem os sintomas causados pela progressão da doença, mas que também reduzam idas aos serviços de emergência e hospitalizações e que garantam suporte no estágio final de vida é fundamental no planejamento dos cuidados paliativos. Idealmente, isso exige a participação de uma equipe multidisciplinar composta por médicos, fisioterapeutas, enfermeiros, psicólogos e assistentes sociais, com conhecimento e preparo apropriados; contudo, o médico responsável com adequada compreensão do quadro do paciente já é capaz de elaborar o manejo da progressão da doença, priorizando o controle dos sintomas.(72)
Em relação ao uso de oxigenoterapia em cuidados paliativos, a indicação requer, além da avaliação das causas passíveis de reversão e de critérios objetivos como a SpO2, uma avaliação global das necessidades do paciente e de um plano terapêutico individualizado. Esse deve ser elaborado de maneira compartilhada entre a equipe, o paciente e os cuidadores.(73)
A ODP poderá aliviar a dispneia se essa estiver associada à hipoxemia, lembrando que a dispneia é uma sensação subjetiva e muitas vezes independente da hipoxemia.(4) O controle de sintomas de pacientes com doenças crônicas avançadas é um recurso terapêutico amplamente discutido. Estudos científicos e recomendações atuais demonstram utilidade limitada da oxigenoterapia em algumas situações.(74) Na prática, observa-se que os benefícios da oxigenoterapia são superestimados, enquanto os seus possíveis riscos e limitações são subestimados. Um estudo observacional com 114 pacientes em fase final de vida não revelou benefício na utilização de oxigênio, não havendo diferença entre a administração de oxigênio ou de ar medicinal para o alívio dos sintomas quando a PaO2 > 55 mmHg. (75) A eventual melhora seria decorrente do contato da face com ar fresco, com estimulação do nervo trigêmeo e diminuição da dispneia; portanto, sem benefício da oxigenoterapia nesse contexto. As diretrizes da British Thoracic Society,(6) por exemplo, recomendam que o uso de oxigênio seja restrito àqueles pacientes com SpO2 < 90% em ar ambiente e que não há papel para o monitoramento rotineiro da SpO2 desde que o paciente esteja confortável nos últimos dias de vida.(76)
Dentre os efeitos colaterais da oxigenoterapia, destacam-se a hipercapnia aguda com efeitos centrais e a lesão pulmonar por estresse oxidativo que ocorre, em geral, com fluxos elevados de oxigênio.(77) O uso do equipamento pode também levar à restrição de atividades, ressecamento das mucosas e desconforto ocasionado por cânulas nasais ou máscaras faciais. (78) As limitações causadas pelo uso da oxigenoterapia devem ser avaliadas criteriosamente pela equipe de saúde multiprofissional, visto que algumas delas podem ter grande impacto na qualidade do fim de vida da pessoa com doença avançada.(74)
O controle da dispneia dos pacientes com doença crônica avançada é baseado na avaliação objetiva da dispneia, aplicação de técnicas de conservação de energia, otimização do tratamento da doença de base e de suas complicações, oxigenoterapia quando houver hipoxemia, reabilitação cardiopulmonar e uso de ventilação não invasiva.(78) O uso de opioide oral, notadamente morfina e di-hidrocodeína, em doses não superiores a 30 mg/dia de morfina ou equivalente, tem sido considerado benéfico na paliação da dispneia, sem risco elevado de depressão respiratória, apesar de seus efeitos adversos como sonolência, náuseas, vômitos e constipação intestinal.(79,80)
ODP NA PÓS-COVID-19 O SARS-CoV-2 infectou e causou a morte de milhões de pessoas no mundo, com grande impacto no sistema de saúde de diversos países, inclusive por falta de fornecimento de oxigênio. Durante a pandemia, alguns conceitos relacionados ao uso do oxigênio foram muito citados e discutidos, como a hipoxemia silenciosa(81-83) e a oxigenoterapia de alto fluxo.(84,85) A hipoxemia silenciosa ocorreu mais em idosos e em portadores de diabetes; nesses pacientes, a resposta hiperventilatória à hipoxemia pode estar atenuada. Uma hipótese ainda não confirmada seria a possível ação direta do vírus no centro respiratório, reduzindo a resposta à hiperventilação. O desvio da curva de dissociação da oxi-hemoglobina para a esquerda nos infectados poderia explicar o fato de alguns pacientes terem maior tolerância à hipoxemia do que outros.(81-83)
Muitos pacientes com a síndrome pós-COVID que evoluíram com sequelas após a alta hospitalar necessitaram ODP. Um estudo apontou que 13,2% dos pacientes que receberam alta hospitalar tiveram indicação de ODP e que essa necessidade foi progressivamente reduzida com a recuperação clínica desses pacientes.(86) Em outro estudo, os fatores de risco relacionados com a necessidade de ODP após a alta hospitalar de pacientes com COVID-19 moderada a grave foram os seguintes: sexo masculino; idade ≥ 50 anos; e ≥ 3 comorbidades, principalmente pneumopatia prévia.(87)
As diretrizes nacionais e internacionais sobre ODP não dispõem de orientações específicas para a alta hospitalar após a infecção por SARS-CoV-2. Uma força-tarefa da European Respiratory Society/American Thoracic Society (ATS)(88) recomenda que pacientes hospitalizados com COVID-19 sejam avaliados quanto à necessidade de suplementação de oxigênio em repouso e durante os esforços, visto que é esperada uma melhora progressiva das trocas gasosas; entretanto, alguns pacientes necessitarão de oxigênio após a alta hospitalar. Outra possibilidade é a presença de dessaturação apenas durante os esforços, devendo-se avaliar a necessidade ou não de suplementação de oxigênio.(88) A detecção da redução da SpO2 justifica a investigação de comorbidades pulmonares e cardiovasculares que não eram conhecidas anteriormente. É recomendada a reavaliação precoce após a alta hospitalar uma vez que a necessidade de ODP pode ser de curta duração.(88)
ODP EM PEDIATRIA As crianças apresentam particularidades relevantes quanto à indicação, manutenção e suspensão da oxigenoterapia. Portanto, as recomendações usadas em adultos não se aplicam às crianças. As principais diferenças da ODP em crianças com relação aos adultos são as seguintes(89-93):
- Devem ser considerados o crescimento físico e o desenvolvimento neurológico.
- A evolução de algumas doenças que cursam com hipoxemia na infância é geralmente favorável; por esse motivo, muitas crianças necessitam ODP apenas por um período limitado de tempo.
- A maioria das condições clínicas é peculiar dessa faixa etária, embora crianças mais velhas e adolescentes possam apresentar indicações semelhantes às dos adultos.
- A indicação e a monitorização do uso do oxigênio são realizadas por oximetria de pulso, e não por gasometria arterial.
- São necessários equipamentos específicos para permitir fluxos baixos de oxigênio.
- Muitas crianças requerem oxigenoterapia apenas durante a noite, sendo necessárias menos horas do que as normalmente indicadas na ODP dos adultos.
- Períodos como atividades físicas (que inclui o banho), sono, e até mesmo as mamadas, podem levar a quedas da saturação; portanto, deve ser individualizado o fornecimento de maior fluxo de oxigênio nessas ocasiões.
- Todas as crianças requerem supervisão de um adulto.
- A oxigenoterapia deve ser fornecida na escola para as crianças em idade escolar.
DBP A definição mais atual de DBP é o diagnóstico por alterações radiográficas persistentes do parênquima pulmonar em recém-nascidos prematuros ≤ 32 semanas de idade gestacional ou com 36 semanas de idade gestacional corrigida que requerem suporte respiratório por três ou mais dias para manter a saturação arterial em 90-95%.(94,95)
A DBP é a indicação mais frequente de ODP em crianças e ocorre em aproximadamente 40% dos recém-nascidos de muito baixo peso (< 1.000 gramas). (93,96-98) Sua incidência não diminuiu ao longo dos anos, justamente pelos avanços importantes nos cuidados neonatais, o que permite cada vez mais a sobrevivência de prematuros extremos.(94-97)
Dentre os benefícios da utilização de ODP, destacam-se melhora do crescimento físico, do desenvolvimento neurológico e do padrão de sono, assim como redução da resistência das vias aéreas, da pressão da artéria pulmonar, do risco de morte súbita e dos despertares noturnos. Além disso, manter a criança junto com a família em casa propicia melhor vínculo emocional e reduz o risco de infecções hospitalares.(89,93)
Indica-se a ODP para o paciente clinicamente estável, que mantenha dependência de oxigênio (SpO2 ≤ 92% em ar ambiente) e não apresente hipercapnia. Dois importantes estudos(99,100) demonstraram que manter a SpO2 > 95% se relacionava a pior desfecho, com necessidade de manter a ODP por mais tempo. Desde então, tem-se evitado manter a SpO2 > 95%. (99,100) Por outro lado, outro estudo(101) comparou a meta de SpO2 de 85-89% com a de 91-95% para crianças nascidas antes de 28 semanas de gestação e demonstrou que a meta de SpO2 < 90% estava associada a um risco aumentado de óbito antes da alta, levando a uma interrupção precoce do estudo. (101) As recomendações atuais são de que a SpO2 fique entre 90% e 95%, sem oscilações frequentes durante o sono ou mamadas. (102,103)
A ODP deve ser considerada para os pacientes com DBP ≥ 36 semanas de idade gestacional corrigida, clinicamente estáveis, e com ganho ponderal ≥ 20 gramas por dia.
Inicia-se com um fluxo de 1-2 L/min, mantendo a SpO2 entre 92% e 95%. A diminuição do oxigênio pode ser considerada após 4 semanas, caso a criança esteja estável e mantendo ganho adequado de peso. Deve-se reduzir o fluxo em 0,25-0,1 L/min, inicialmente no período que a criança esteja acordada, desde que a SpO2 permaneça ≥ 92%.(97)
FC A indicação da ODP na FC deve ser individualizada, e, de modo geral, crianças e adolescentes devem recebê-la via cânula nasal, respeitando adaptações pertinentes, como no caso de pacientes com traqueostomia.(104) A oxigenoterapia reduzirá a dispneia e retardará o surgimento de cor pulmonale. Escolares e adolescentes com PaO2 ≤ 55 mmHg ou SpO2 ≤ 88% devem receber oxigenoterapia com o menor fluxo possível para manter SpO2 > 90%.(90,104) Publicações recentes da ATS recomendam ponderar sobre a prescrição da ODP para pacientes pediátricos com FC que mantenham saturação em 90-93% mas com dispneia aos esforços. (89,90) A prescrição para lactentes e pré-escolares estará indicada para se manter a SpO2 ≥ 93%, de forma semelhante aos pacientes com DBP.(92)
Desmame da ODP em pediatria O desmame da ODP poderá ocorrer com o crescimento e a maturação pulmonar, e a possível melhora do agravo pulmonar. O médico deve avaliar o paciente clinicamente e assegurar-se de que o desmame é viável pelas medidas da SpO2.(89,90) O desmame será programado semanalmente com redução gradual do fluxo de oxigênio ou pela retirada da ODP em períodos do dia de forma crescente, mantendo as visitas médicas semanais a mensais para a garantia do desmame de forma segura.(89) Os lactentes recebendo fluxos de até 0,1 L/min, os pré-escolares recebendo fluxos de 0,1 a 0,25 L/min e as crianças maiores recebendo fluxos de 0,25 a 0,5 L/min podem estar aptas a descontinuar a ODP. Após a retirada do oxigênio, é fortemente sugerida a realização de um estudo de oximetria noturna com aparelho pediátrico apropriado.(89,90) Os equipamentos utilizados na ODP devem permanecer na residência do paciente por um período de tempo que garanta sua segurança(89); a recomendação nacional sugere por no mínimo 3 meses após a suspensão da ODP.(92) Em seguida, o controle de oximetria deverá ser realizado em duas ocasiões com intervalo de um mês e, se essa se mantiver adequada, o oxigênio e os equipamentos poderão ser retirados da residência do paciente.(92)
ODP EM PACIENTES COM DISTÚRBIOS RESPIRATÓRIOS DO SONO Os distúrbios respiratórios do sono são caracterizados por eventos respiratórios repetitivos relacionados ao sono, cursando com hipoxemia intermitente e fragmentação do sono, abrangendo a AOS, a apneia central do sono (ACS) e a hipoventilação relacionada ao sono, sendo a AOS o distúrbio mais prevalente.(105)
A intensidade e a frequência de hipoxemia intermitente durante os episódios recorrentes de apneias/hipopneias durante o sono comumente levam a consequências cardiovasculares, metabólicas e neurocognitivas, impactando na morbidade e na mortalidade.(106)
A pressão positiva nas vias aéreas (PAP) constitui a terapia padrão por manter a patência da via aérea superior e corrigir a hipoxemia intermitente.(105,107) Contudo, apesar de ser um tratamento extremamente eficaz, a adesão é limitada.(106,107,108)
AOS Uma revisão sistemática e meta-análise avaliando ECR mostrou a superioridade da CPAP em comparação com o uso de oxigênio noturno na redução do índice de apneia-hipopneia (IAH) em indivíduos com AOS.(109) Entretanto, estudos prévios documentaram o prolongamento da duração dos eventos respiratórios obstrutivos durante o uso de oxigênio noturno.(38,110) Em uma recente meta-análise, a oxigenoterapia suplementar foi menos eficaz na redução do IAH, do tempo de SpO2 < 90% e da pressão arterial sistêmica, assim como na melhora da qualidade do sono em comparação com CPAP.(111) O oxigênio pode ser utilizado conjuntamente à terapia com PAP quando a SpO2 persistir ≤ 88% por pelo menos 5 min, apesar da titulação adequada e total controle dos eventos obstrutivos (iniciar oxigênio a 1 L/min e titular para manter a SpO2 em 88-94%).(112)
ACS Estudos prévios relataram o efeito benéfico da suplementação de oxigênio na ACS associada à respiração de Cheyne-Stokes em indivíduos com insuficiência cardíaca congestiva (ICC).(113,114) Duas meta-análises compararam o efeito de CPAP, servoventilação adaptativa (SVA) e suplementação de oxigênio no IAH e na fração de ejeção do ventrículo esquerdo (FEVE) em pacientes com ICC e ACS associada à respiração de Cheyne-Stokes.(115,116) A primeira meta-análise(115) com 919 pacientes demonstrou que a SVA tinha maior probabilidade de reduzir o IAH, seguida por suplementação de oxigênio e CPAP. Na segunda meta-análise,(116) com a inclusão de 951 pacientes, verificou-se que CPAP e SVA foram igualmente eficazes na melhora da FEVE, o que não ocorreu com o uso de oxigênio noturno. Embora a SVA possa melhorar o IAH e a FEVE, um estudo observou aumento da mortalidade em indivíduos com ICC-ACS e FEVE ≤ 45%.(117) A oxigenoterapia noturna pode não eliminar os eventos obstrutivos que frequentemente coexistem com os eventos centrais em pacientes com ICC.(118) Na ACS, o oxigênio noturno reduz efetivamente o IAH secundário às ACS e melhora a SpO2, podendo servir como alternativa à terapia com PAP.(108,119) No entanto, faltam estudos de longo prazo para avaliar o impacto da ODP na ACS.
Síndrome de sobreposição DPOC e AOS A síndrome de sobreposição DPOC e AOS cursa com hipoxemia noturna mais intensa do que a AOS ou a DPOC isoladamente, levando a pior prognóstico.(120,121) A oxigenoterapia noturna pode ser indicada quando a hipóxia noturna persiste nos pacientes com DPOC, apesar do tratamento adequado.(38,108,110) Contudo, por suprimir o impulso respiratório hipóxico, o oxigênio pode contribuir para o prolongamento da duração da apneia, levando a hipercapnia e acidose em pacientes com AOS, especialmente na síndrome de sobreposição ou na hipoventilação.(38,108,110) O tratamento atual de pacientes com sobreposição inclui terapia regular com CPAP, observando-se que CPAP está indicada na AOS de grau acentuado ou moderado quando associada a sintomas ou hipoxemia noturna significativa. Não há indicação de CPAP na OAS leve.(120)
Em estudos observacionais, os pacientes com sobreposição DPOC e AOS tratados com CPAP tiveram taxas de sobrevida comparáveis àquelas de pacientes apenas com DPOC, enquanto aqueles com essa sobreposição não tratados com CPAP exibiram mortalidade mais elevada.(121)
Síndrome de hipoventilação por obesidade A síndrome de hipoventilação por obesidade (SHO) constitui a tríade obesidade, anormalidade das trocas gasosas (hipercapnia) e exclusão de outras causas de hipoventilação. Em relação ao tratamento, as publicações mais recentes da ATS e da European Respiratory Society recomendam que o uso de CPAP, mais do que BiPAP, seja oferecido como primeira linha de tratamento para pacientes ambulatoriais com SHO e AOS grave, estando essa associação presente em mais de 70% dos pacientes com SHO.(122,123) No entanto, a ventilação não invasiva é preferida na minoria dos pacientes de SHO sem AOS ou com formas mais leves de AOS (aproximadamente < 30%). A oxigenoterapia isolada na SHO deve ser evitada devido ao efeito prejudicial na ventilação e ao risco de precipitar insuficiência respiratória hipercápnica.(123)
SUPLEMENTAÇÃO DE OXIGÊNIO NO EXERCÍCIO E NA REABILITAÇÃO PULMONAR A oxigenação tecidual depende de uma sequência de fenômenos que acontecem desde a captação de oxigênio do ar atmosférico para os pulmões, seguida da oferta adequada de oxigênio para a periferia, por meio do transporte de hemoglobina e fluxo sanguíneo adequado, até a entrega para a maquinaria energética mitocondrial para a síntese aeróbica de ATP e sua utilização pelos músculos.(124)
Os principais efeitos da suplementação de oxigênio durante o exercício na DPOC e na DPI são os seguintes: efeito central, com prevenção da redução da oxigenação cerebral; efeito ventilatório, com menor impulso ventilatório por menor estímulo do quimiorreceptor carotídeo e redução da hiperinsuflação dinâmica; efeito cardiocirculatório, pela vasodilatação pulmonar, aumento do DC e diminuição da pressão da artéria pulmonar; e efeito muscular, com redução da disfunção muscular, menor produção de ácido lático e diminuição da ativação dos metaboergoreceptores musculares, diminuindo o estímulo ventilatório.(125-127) A modulação desses mecanismos tem o potencial de aliviar os sintomas como a dispneia e a fadiga, além de melhorar a qualidade de vida e a capacidade de exercício, principalmente durante a reabilitação. Entretanto, dados controversos ainda são descritos na literatura em relação à suplementação de oxigênio, especialmente para pacientes normoxêmicos com ou sem hipoxemia induzida pelo esforço.
DPOC A hipoxemia induzida pelo exercício é comum na DPOC, estando presente em quase metade dos pacientes encaminhados para reabilitação pulmonar (RP). Em geral, esses pacientes não toleram exercícios de alta intensidade, muitas vezes sendo necessário diminuir a intensidade do treinamento, o que poderia limitar a eficácia da RP.(128) Na avaliação da suplementação aguda de oxigênio, 124 pacientes com DPOC moderada a grave, divididos em normoxêmicos e em hipoxêmicos em repouso ou somente durante o exercício, foram submetidos a um teste de caminhada de seis minutos (TC6) com oxigênio suplementar ou com ar comprimido. (129) Os dois grupos hipoxêmicos se beneficiaram do oxigênio suplementar por cateter nasal (CN), com aumento da capacidade de exercício, contudo sem atingir diferença clinicamente significante (> 30 m).(129) Ao se comparar o efeito da suplementação de oxigênio com a de ar comprimido durante o treinamento de pacientes normoxêmicos sem hipoxemia induzida pelo exercício (n = 29), a suplementação de oxigênio permitiu maior intensidade de treinamento e maior capacidade de exercício (endurance em cicloergômetro: 14,5 min vs. 10,5 min; p < 0,05) durante um programa de RP.(130) É interessante notar que os respondedores são aqueles com maior dessaturação(131) e menor capacidade de exercício(132) na avaliação basal ou que apresentaram ganho > 10% na distância percorrida na avaliação basal após a suplementação de oxigênio.(132)
Ao contrário desses resultados, um estudo multicêntrico com 111 participantes com DPOC moderada a grave e hipoxemia induzida durante o TC6 (SpO2 < 90%), a suplementação de oxigênio não resultou em aumento da capacidade de exercício ou da qualidade de vida quando comparada à suplementação de ar comprimido. Vale ressaltar que ambos os grupos obtiveram benefícios após o treinamento físico, com aumentos significativos na capacidade de exercício e na qualidade de vida.(133) Entretanto, questiona-se se o nível de intensidade proposto pelo estudo para o treinamento daqueles pacientes foi de fato alcançado. (134) Considerando-se pacientes com DPOC grave a muito grave e com hipoxemia em repouso (n = 50), foi avaliada a suplementação de oxigênio por meio de um sistema de fluxo constante vs. titulação automática de oxigênio.(135) Essa última acarretou melhor oxigenação, maior tempo de endurance ao caminhar, maior oxigenação observada por SpO2 e PaO2, menor sensação de dispneia e sem impacto na PaCO2. Os respondedores à titulação automática de oxigênio tenderam a apresentar menor valor de lactato, menos fadiga em membros inferiores no final do teste de endurance e menor sensação de dispneia. (135) Já em relação ao treinamento com máscara de Venturi ou CN de alto fluxo (CNAF), ambos os grupos obtiveram benefícios do programa de treinamento físico com melhora significativa na capacidade de exercício, sintomas e qualidade de vida.(136)
Numa revisão sistemática e meta-análise recente, na qual foram incluídos 7 estudos para a avaliação de oxigênio suplementar e RP, foi demonstrado que a suplementação de oxigênio durante a RP não melhorou a capacidade de exercício, os escores de dispneia ou a qualidade de vida, mas com a ressalva de nível de evidência fraca, principalmente pela intervenção heterogênea nos estudos.(137)
Apesar dos resultados contraditórios, de acordo com as diretrizes internacionais, os pacientes que recebem ODP devem continuar com a suplementação de oxigênio durante o treinamento físico e elevar o fluxo à medida que a demanda de oxigênio aumente durante o exercício.(6,20,25) Em alguns casos, deve ser considerada uma avaliação formal que demonstre melhora na tolerância ao exercício com testes de suplementação aguda de oxigênio.(6,20,25)
DPI Pacientes com DPI têm redução da tolerância ao exercício avaliada por TC6, consumo máximo de oxigênio e tempo de endurance. Uma menor capacidade de exercício tem relação com pior sobrevida. Na avaliação de suplementação de oxigênio aguda, pacientes com DPI leve a moderada (n = 72) com hipoxemia induzida por exercício, comparando-se à suplementação de oxigênio com a de ar comprimido, houve aumento no tempo de endurance, menor dessaturação e menos sintomas com o uso de oxigênio.(138) Foram considerados respondedores aqueles que atingiram um menor nadir de SpO2 no TC6 na avaliação basal com ar comprimido(138); resultado semelhante foi encontrado comparando-se o valor de FiO2 = 50% com a suplementação de ar comprimido.(139)
Sabe-se que pacientes com DPI podem ter dessaturação significativa, atingindo valores muito baixos (SpO2 < 80% no esforço), nem sempre sendo possível manter a SpO2 > 90% com oxigênio via CN. Comparando-se à suplementação de oxigênio de diferentes CN com uma CN com reservatório com um pendente interno incorporado ao seu lúmen (Oxymizer; Drive DeVilbiss Healthcare, Port Washington, NY, EUA) em pacientes em oxigenoterapia ambulatorial (n = 21), houve melhora da tolerância ao exercício, mas sem impacto na dispneia.(140) Apesar da melhora na oxigenação, ela não foi sustentada durante o exercício mesmo com o uso do Oxymizer.(140) Nesse sentido, um estudo com pacientes com DPI grave (n = 25) testados em ar ambiente, com suplementação de oxigênio via CN a 4 L/min ou via CNAF com FiO2 = 50% a 30-50 L/min (aquecido a 34°C e umidificado), demonstrou que o uso da CNAF aumentou o tempo de endurance comparado ao dos outros dois grupos, além de ser associado com menor cinética de dessaturação de oxigênio, menor resposta cronotrópica e menor sensação de dispneia e de fadiga nas pernas.(141) A hiperóxia (FiO2 = 30-60%), comparada a FiO2 = 21%, também demonstrou aumento no tempo de endurance, menor ventilação, menor sensação de dispneia(142) e melhora significativa da oxigenação muscular avaliada pela fadigabilidade, com diminuição da sensação de desconforto nas pernas ao esforço.(127)
Embora a capacidade de exercício tenha sido aumentada, uma revisão sistemática avaliando o impacto do oxigênio suplementar nos resultados do treinamento em pacientes com DPI mostrou que a oxigenoterapia não tem efeito na dispneia durante o exercício.(57) Uma vez que altas taxas de fluxo de oxigênio são frequentemente necessárias nesse grupo de pacientes, é importante selecionar os dispositivos de fornecimento de oxigênio apropriados (por exemplo, CN ou máscara facial simples), e, quando uma concentração maior de oxigênio for necessária, outros dispositivos deverão ser avaliados, como máscaras não reinalantes ou CNAF, essa última indicada de acordo com protocolos institucionais.
HP O benefício e a segurança da RP em pacientes com HP têm sido reportados principalmente nos últimos 15 anos. Nas diretrizes para RP em HP,(143) baseadas nos protocolos publicados, a oferta de oxigênio foi realizada de acordo com a indicação e a necessidade do paciente, e a dessaturação foi considerada evento adverso em 16 de 674 pacientes incluídos (2,4%). Na avaliação de 519 pacientes incluídos em diferentes estudos, o treinamento físico em média foi baseado em ~60% da FC máxima, não devendo exceder 120 bpm, e SpO2 > 85-90%. A dessaturação de oxigênio < 85-90% ou FC > 120 bpm foram usadas como critérios limitantes para ajustar a intensidade do treinamento, acarretando em interrupção precoce do exercício ou redução da intensidade do treinamento.(144,145)
OXIGÊNIO EM VIAGENS AÉREAS As aeronaves comerciais podem atingir altitude de até 45.000 pés (13.716 m), com grande redução da pressão barométrica e da PaO2.(146) Para que os voos sejam possíveis, as cabines são pressurizadas a 8.000 pés (2.438 m) e, nessas condições, a FiO2 é equivalente a 15,1% em relação ao nível do mar; num indivíduo sadio, dependendo da idade e da ventilação minuto, sua PaO2 e a SaO2 reduziriam para 60-75 mmHg e 89-94%, respectivamente.(146-148)
Para manter a PaO2 adequada em situações de hipóxia hipobárica, há aumento da ventilação minuto, da FC e do DC, além de vasoconstrição pulmonar com redistribuição do fluxo para as regiões apicais, alterando a V/Q. A maioria dos indivíduos tolera bem essas modificações, mas alguns poderão apresentar dispneia, sonolência, alterações cognitivas, lipotimia e dor torácica.
Pacientes com pneumopatias crônicas, principalmente aqueles em ODP ou com SpO2 limítrofe, e portadores de outras doenças que cursem com hipoxemia terão a mesma agravada e poderão apresentar manifestações clínicas durante os voos.(147-150) Dessa forma, pacientes com risco de hipoxemia em viagens aéreas devem ser avaliados quanto à necessidade de suplementação de oxigênio. O uso de ODP, a presença de comorbidades e a informação de sintomas respiratórios em voos prévios, tais como dispneia, tosse ou dor torácica, devem ser investigados. Os pacientes devem viajar apenas nas fases estáveis das suas doenças.(148) Além disso, durante os voos, os passageiros podem permanecer imobilizados por longos períodos e estarem expostos a baixas temperaturas e ressecamento do ar, fatores que potencializam o risco de exacerbações ou outras complicações como tromboembolia venosa, o que reforça a importância de permanecer com SpO2 adequada durante a viagem.(151)
Pacientes com SpO2 > 95% em ar ambiente podem voar sem suplementação; por outro lado, aqueles com SpO2 ≤ 92% devem receber oxigênio suplementar durante as viagens aéreas. Os pacientes com SpO2 entre 92% e 95% devem ser submetidos a TC6 ou teste de simulação de hipóxia em altitude, esse último raramente está disponível em nosso meio. Pacientes que persistirem com SpO2 ≤ 84% durante um desses testes também necessitarão de suplementação de oxigênio durante o voo.(149,150) O teste de simulação de hipóxia em altitude simula as condições ambientais (pressão barométrica e FiO2) encontradas nas cabines pressurizadas. Idealmente, deveria ser realizado em câmaras hipobáricas; porém, essas câmaras são pouco disponíveis, e o teste, quando realizado em câmaras normobáricas, não tem resultados confiáveis.(149,150)
Pacientes que necessitem um fluxo > 4 L/min para corrigir a hipoxemia devem ser desencorajados a voar e, caso o façam, devem utilizar transporte aeromédico. (151) É importante salientar que essas recomendações se baseiam principalmente em estudos realizados em pacientes com diagnóstico de DPOC e são extrapolados para outras doenças respiratórias.(152,153)
Após uma avaliação, o médico assistente deve preencher a autorização de voo com a prescrição do fluxo de oxigênio e de outras medidas necessárias no documento conhecido como Medical Information Form (formulário de informações médicas), que é fornecido pelas empresas aéreas. O preenchimento deve ocorrer pelo menos 72 h antes do voo para que haja tempo hábil de apreciação pela empresa. Os pacientes devem programar com antecedência suas viagens aéreas, pois o tempo para liberação da companhia é variável.(151-153)
Na aeronave, a suplementação pode ser realizada por oxigênio fornecido pela companhia aérea (cilindro ou concentrador de oxigênio) ou pelo concentrador do paciente, desde que homologado. No período de trânsito e permanência nos aeroportos o paciente usará o seu concentrador. Recentemente, a Agência Nacional de Aviação Civil divulgou uma instrução complementar sobre o uso de concentradores portáteis em aeronaves comerciais.(154) Os pontos mais importantes são os seguintes: os concentradores devem ser de marcas autorizadas para voo, o equipamento e as baterias não podem ser despachados, e as baterias devem garantir autonomia do equipamento de no mínimo 150% do tempo do voo. Infelizmente, as empresas aéreas que operam comercialmente não têm regras homogêneas, nem em relação ao formato do formulário, nem em relação ao fornecimento de oxigênio. É necessário que o médico assistente consulte os procedimentos de cada companhia para a orientação adequada do paciente (Quadro 9 e Figura 1).
CUIDADOS GERAIS PARA PRESCRIÇÃO DE ODP Alguns cuidados devem ser observados na prescrição de ODP. Em relação aos pacientes, atentar para educação continuada e treinamento relacionados ao equipamento de oxigênio do paciente, segurança no uso e autogerenciamento. Entre as responsabilidades de quem prescreve, destacam-se: a) determinação do objetivo e da necessidade da ODP por meio de gasometria arterial; b) preenchimento correto dos relatórios e atenção aos protocolos municipais ou estaduais; c) seleção de um fornecedor qualificado de equipamentos médicos duráveis; d) titulação do oxigênio em diferentes momentos (repouso, atividades da vida diária, sono, esforços/exercício, viagens, exacerbações) com o objetivo de determinar o fluxo adequado para que o paciente sempre mantenha a SpO2 > 90%; e) testar o fluxômetro, pois o equipamento pode fornecer fluxo diferente do que está indicando; f) prescrição de fluxos adequados de oxigênio para cada situação específica, duração mínima do uso, fontes diversas para fornecimento de oxigênio e acessórios necessários; g) reavaliação periódica da necessidade de ODP para renovação e/ou modificação de prescrição; e h) educação dos pacientes e de seus familiares sobre o uso correto da ODP com enfoque especial sobre a boa adesão. Essas recomendações estão descritas em diversos quadros deste documento: sobre aspectos práticos da prescrição de oxigênio (Quadros 1-7), sobre o protocolo para prescrição de oxigênio (Quadro 8) e sobre viagens aéreas (Quadro 9 e Figura 1).
CONSIDERAÇÕES FINAIS A ODP teve seu uso difundido a partir da década de 1980. Mesmo com poucos estudos e com várias perguntas sem respostas, houve rápida divulgação dos benefícios da ODP, e várias sociedades de pneumologia em todo mundo passaram a orientar o seu uso. As recomendações neste documento refletem uma integração de evidências atuais e das previamente estabelecidas, sendo a ODP prescrita para pacientes com hipoxemia grave em repouso com o objetivo de melhorar a sobrevida e a qualidade de vida, apoiadas por estudos em pacientes com DPOC. As evidências existentes sugerem não prescrever ODP para pacientes com DPOC com hipoxemia moderada em repouso. A prescrição de oxigênio para pacientes com DPI com hipoxemia grave em repouso é fortemente recomendada. Ainda faltam evidências sobre o papel da ODP em outras doenças pulmonares, como a HP, e sobre o uso durante o sono e durante atividades físicas. Pesquisas futuras são fundamentais para a avaliação sobre a segurança da decisão compartilhada entre os pacientes e os seus médicos sobre ODP e a melhor abordagem para a descontinuação da ODP naqueles sem hipoxemia grave em repouso.
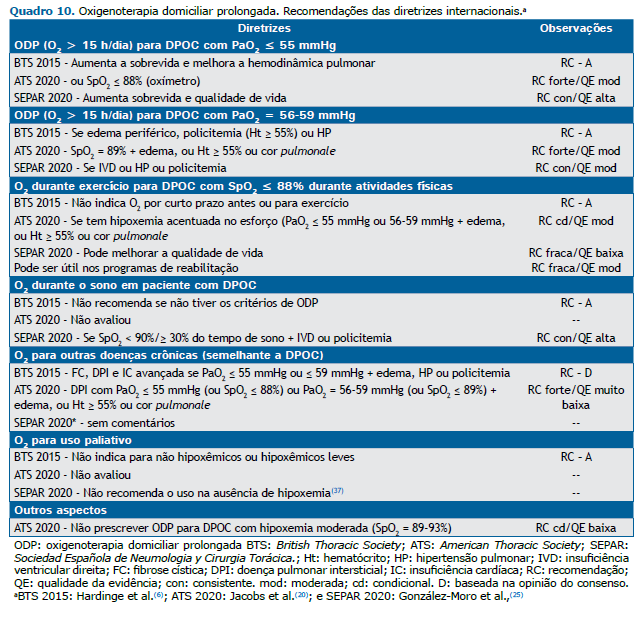
É necessário destacar que os programas de ODP têm custos elevados e que sua prescrição deve ser correta para que os pacientes realmente se beneficiem da mesma e obtenham o resultado esperado, tanto do ponto de vista médico quanto do ponto de vista social, laborativo e familiar.(6,19,20,25) Os autores analisaram as recomendações das três últimas diretrizes internacionais sobre ODP e, após uma análise pormenorizada dessas,(6,20,25) tendo suas principais conclusões resumidas no Quadro 10, concordaram com as mesmas indicações, mas enfatizando a necessidade de mais estudos, principalmente em relação às doenças crônicas além da DPOC.
CONTRIBUIÇÕES DOS AUTORES Todos os autores participaram de todas as etapas de elaboração do documento: planejamento, redação, revisão e aprovação final do manuscrito.
CONFLITOS DE INTERESSE Nenhum declarado.
REFERÊNCIAS 1. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391-398. https://doi.org/10.7326/0003-4819-93-3-391
2. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681-686. https://doi.org/10.1016/S0140-6736(81)91970-X
3. Timms RM, Khaja FU, Williams GW. Hemodynamic response to oxygen therapy in chronic obstructive pulmonary disease. Ann Intern Med. 1985;102(1):29-36. https://doi.org/10.7326/0003-4819-102-1-29
4. McDonald CF, Whyte K, Jenkins S, Serginson J, Frith P. Clinical Practice Guideline on Adult Domiciliary Oxygen Therapy: Executive summary from the Thoracic Society of Australia and New Zealand. Respirology. 2016;21(1):76-78. https://doi.org/10.1111/resp.12678
5. Lacasse Y, Bernard S, Maltais F. Eligibility for home oxygen programs and funding across Canada. Can Respir J. 2015;22(6):324-330. https://doi.org/10.1155/2015/280604
6. Hardinge M, Suntharalingam J, Wilkinson T; British Thoracic Society. Guideline update: The British Thoracic Society Guidelines on home oxygen use in adults. Thorax. 2015;70(6):589-591. https://doi.org/10.1136/thoraxjnl-2015-206918
7. Ferguson GT, Cherniack RM. Management of chronic obstructive pulmonary disease. N Engl J Med. 1993;328(14):1017-1022. https://doi.org/10.1056/NEJM199304083281408
8. Doherty DE, Petty TL, Bailey W, Carlin B, Cassaburi R, Christopher K, et al. Recommendations of the 6th long-term oxygen therapy consensus conference. Respir Care. 2006;51(5):519-525.
9. Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373-378. https://doi.org/10.1164/rccm.200507-1161WS
10. Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191. https://doi.org/10.7326/0003-4819-155-3-201108020-00008
11. Petty TL, Bliss PL. Ambulatory oxygen therapy, exercise, and survival with advanced chronic obstructive pulmonary disease (the Nocturnal Oxygen Therapy Trial revisited). Respir Care. 2000;45(2):204-213.
12. Crockett AJ, Cranston JM, Moss JR, Alpers JH. A review of long-term oxygen therapy for chronic obstructive pulmonary disease. Respir Med. 2001;95(6):437-443. https://doi.org/10.1053/rmed.2001.1064
13. Eaton T, Lewis C, Young P, Kennedy Y, Garrett JE, Kolbe J. Long-term oxygen therapy improves health-related quality of life. Respir Med. 2004;98(4):285-293. https://doi.org/10.1016/j.rmed.2003.10.008
14. Wedzicha JA. Effects of long-term oxygen therapy on neuropsychiatric function and quality of life. Respir Care. 2000;45(1):119-126.
15. Ringbaek TJ, Viskum K, Lange P. Does long-term oxygen therapy reduce hospitalisation in hypoxaemic chronic obstructive pulmonary disease?. Eur Respir J. 2002;20(1):38-42. https://doi.org/10.1183/09031936.02.00284202
16. Morrison DA, Stovall JR. Increased exercise capacity in hypoxemic patients after long-term oxygen therapy. Chest. 1992;102(2):542-550. https://doi.org/10.1378/chest.102.2.542
17. Weitzenblum E, Sautegeau A, Ehrhart M, Mammosser M, Pelletier A. Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985;131(4):493-498. https://doi.org/10.1164/arrd.1985.131.4.493
18. Zieliński J, Tobiasz M, Hawryłkiewicz I, Sliwiński P, Pałasiewicz G. Effects of long-term oxygen therapy on pulmonary hemodynamics in COPD patients: a 6-year prospective study. Chest. 1998;113(1):65-70. https://doi.org/10.1378/chest.113.1.65
19. Sociedade Brasileira de Pneumologia e Tisiologia. Oxigenioterapia domiciliar prolongada (ODP). J Pneumol. 2000;26(6):341-350. https://doi.org/10.1590/S0102-35862000000600011
20. Jacobs SS, Krishnan JA, Lederer DJ, Ghazipura M, Hossain T, Tan AM, et al. Home Oxygen Therapy for Adults with Chronic Lung Disease. An Official American Thoracic Society Clinical Practice Guideline [published correction appears in Am J Respir Crit Care Med. 2021 Apr 15;203(8):1045-1046]. Am J Respir Crit Care Med. 2020;202(10):e121-e141. https://doi.org/10.1164/rccm.202009-3608ST
21. Collins JA, Rudenski A, Gibson J, Howard L, O’Driscoll R. Relating oxygen partial pressure, saturation and content: the haemoglobin-oxygen dissociation curve. Breathe (Sheff). 2015 Sep;11(3):194-201. https://doi.org/10.1183/20734735.001415
22. Carvalho CRR. Fisiopatologia Respiratória. São Paulo: Atheneu; 2005.
23. Wagner PD. The physiological basis of pulmonary gas exchange: implications for clinical interpretation of arterial blood gases. Eur Respir J. 2015 Jan;45(1):227-43. https://doi.org/10.1183/09031936.00039214
24. Petersson J, Glenny RW. Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J. 2014 Oct;44(4):1023-41. https://doi.org/10.1183/09031936.00037014
25. González-Moro JMR, Quiroga LB, Navarrete BA, Michavila IA, Lobato SD. Oxigenoterapia continua domiciliaria [Article in Spanish]. Open Respir Arch. 2020;2(2):33-45. https://doi.org/10.1016/j.opresp.2020.03.004
26. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012 Feb 3;148(3):399-408. https://doi.org/10.1016/j.cell.2012.01.021
27. West JB. Physiological Effects of Chronic Hypoxia. N Engl J Med. 2017;376(20):1965-1971. https://doi.org/10.1056/NEJMra1612008
28. Wan J, Lata C, Santilli A, Green D, Roy S, Santilli S. Supplemental oxygen reverses hypoxia-induced smooth muscle cell proliferation by modulating HIF-alpha and VEGF levels in a rabbit arteriovenous fistula model. Ann Vasc Surg. 2014;28(3):725-736. https://doi.org/10.1016/j.avsg.2013.10.007
29. Chaouat A, Weitzenblum E, Kessler R, Charpentier C, Enrhart M, Schott R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J. 1999;14(5):1002-1008. https://doi.org/10.1183/09031936.99.14510029
30. Górecka D, Gorzelak K, Sliwiński P, Tobiasz M, Zieliński J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax. 1997;52(8):674-679. https://doi.org/10.1136/thx.52.8.674
31. Long-Term Oxygen Treatment Trial Research Group, Albert RK, Au DH, Blackford AL, Casaburi R, Cooper JA Jr, et al. A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation. N Engl J Med. 2016;375(17):1617-1627. https://doi.org/10.1056/NEJMoa1604344
32. Haidl P, Clement C, Wiese C, Dellweg D, Köhler D. Long-term oxygen therapy stops the natural decline of endurance in COPD patients with reversible hypercapnia. Respiration. 2004;71(4):342-347. https://doi.org/10.1159/000079637
33. Heaton RK, Grant I, McSweeny AJ, Adams KM, Petty TL. Psychologic effects of continuous and nocturnal oxygen therapy in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1983;143(10):1941-1947. https://doi.org/10.1001/archinte.1983.00350100121023
34. Clini E, Vitacca M, Foglio K, Simoni P, Ambrosino N. Long-term home care programmes may reduce hospital admissions in COPD with chronic hypercapnia. Eur Respir J. 1996;9(8):1605-1610. https://doi.org/10.1183/09031936.96.09081605
35. Guyatt GH, Nonoyama M, Lacchetti C, Goeree R, McKim D, Hells-Ansdell D, et al. A randomized trial of strategies for assessing eligibility for long-term domiciliary oxygen therapy. Am J Respir Crit Care Med. 2005;172(5):573-580. https://doi.org/10.1164/rccm.200412-1692OC
36. Timms RM, Kvale PA, Anthonisen NR, Boylen CT, Cugell DW, Petty TL, et al. Selection of patients with chronic obstructive pulmonary disease for long-term oxygen therapy. JAMA. 1981;245(24):2514-2515. https://doi.org/10.1001/jama.1981.03310490032020
37. Abdo WF, Heunks LM. Oxygen-induced hypercapnia in COPD: myths and facts. Crit Care. 2012;16(5):323. https://doi.org/10.1186/cc11475
38. Alford NJ, Fletcher EC, Nickeson D. Acute oxygen in patients with sleep apnea and COPD. Chest. 1986;89(1):30-38. https://doi.org/10.1378/chest.89.1.30
39. Carlos WG, Baker MS, McPherson KA, Bosslet GT, Sood R, Torke AM. Smoking-Related Home Oxygen Burn Injuries: Continued Cause for Alarm. Respiration. 2016;91(2):151-155. https://doi.org/10.1159/000443798
40. Avdeev SN, Aisanov ZR, Chuchalin AG. Compliance as a critical issue in long-term oxygen therapy. Monaldi Arch Chest Dis. 1999;54(1):61-66.
41. Cullen DL, Stiffler D. Long-term oxygen therapy: review from the patients’ perspective. Chron Respir Dis. 2009;6(3):141-147. https://doi.org/10.1177/1479972309103046
42. Jacobs SS, Lindell KO, Collins EG, Garvey CM, Hernandez C, McLaughlin S et al. Patient Perceptions of the Adequacy of Supplemental Oxygen Therapy. Results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15(1):24-32. https://doi.org/10.1513/AnnalsATS.201703-209OC
43. Oba Y, Salzman GA, Willsie SK. Reevaluation of continuous oxygen therapy after initial prescription in patients with chronic obstructive pulmonary disease. Respir Care. 2000;45(4):401-406.
44. Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519-2531. https://doi.org/10.1016/S0140-6736(16)00576-6
45. Elphick HE, Mallory G. Oxygen therapy for cystic fibrosis. Cochrane Database Syst Rev. 2013;2013(7):CD003884. https://doi.org/10.1002/14651858.CD003884.pub4
46. Zinman R, Corey M, Coates AL, Canny GJ, Connolly J, Levison H, et al. Nocturnal home oxygen in the treatment of hypoxemic cystic fibrosis patients. J Pediatr. 1989;114(3):368-377. https://doi.org/10.1016/S0022-3476(89)80553-0
47. Kapnadak SG, Dimango E, Hadjiliadis D, Hempstead SE, Tallarico E, Pilewski JM, et al. Cystic Fibrosis Foundation consensus guidelines for the care of individuals with advanced cystic fibrosis lung disease. J Cyst Fibros. 2020;19(3):344-354. https://doi.org/10.1016/j.jcf.2020.02.015
48. Khor YH, Renzoni EA, Visca D, McDonald CF, Goh NSL. Oxygen therapy in COPD and interstitial lung disease: navigating the knowns and unknowns. ERJ Open Res. 2019;5(3):00118-2019. https://doi.org/10.1183/23120541.00118-2019
49. Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760-1769. https://doi.org/10.1016/S0140-6736(11)60405-4
50. King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis [published correction appears in N Engl J Med. 2014 Sep 18;371(12):1172]. N Engl J Med. 2014;370(22):2083-2092. https://doi.org/10.1056/NEJMoa1402582
51. Khor YH, Goh NS, Glaspole I, Holland AE, McDonald CF. Exertional Desaturation and Prescription of Ambulatory Oxygen Therapy in Interstitial Lung Disease. Respir Care. 2019;64(3):299-306. https://doi.org/10.4187/respcare.06334
52. Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long QI, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168(9):1084-1090. https://doi.org/10.1164/rccm.200302-219OC
53. Lettieri CJ, Nathan SD, Browning RF, Barnett SD, Ahmad S, Shorr AF. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med. 2006;100(10):1734-1741. https://doi.org/10.1016/j.rmed.2006.02.004
54. Du Plessis JP, Fernandes S, Jamal R, Camp P, Johannson K, Schaeffer M, et al. Exertional hypoxemia is more severe in fibrotic interstitial lung disease than in COPD. Respirology. 2018;23(4):392-398. https://doi.org/10.1111/resp.13226
55. Vainshelboim B, Kramer MR, Izhakian S, Lima RM, Oliveira J. Physical Activity and Exertional Desaturation Are Associated with Mortality in Idiopathic Pulmonary Fibrosis. J Clin Med. 2016;5(8):73. https://doi.org/10.3390/jcm5080073
56. Troy LK, Young IH, Lau EMT, Wong KKH, Yee BJ, Torzillo PJ, et al. Nocturnal hypoxaemia is associated with adverse outcomes in interstitial lung disease. Respirology. 2019;24(10):996-1004. https://doi.org/10.1111/resp.13549
57. Bell EC, Cox NS, Goh N, Glaspole I, Westall GP, Watson A, et al. Oxygen therapy for interstitial lung disease: a systematic review. Eur Respir Rev. 2017;26(143):160080. https://doi.org/10.1183/16000617.0080-2016
58. Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society [published correction appears in Thorax. 2008 Nov;63(11):1029. multiple author names added]. Thorax. 2008;63 Suppl 5:v1-v58. https://doi.org/10.1136/thx.2008.101691
59. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. https://doi.org/10.1164/rccm.2009-040GL
60. Cottin V, Crestani B, Valeyre D, Wallaert B, Cadranel J, Dalphin JC, et al. Diagnosis and management of idiopathic pulmonary fibrosis: French practical guidelines. Eur Respir Rev. 2014;23(132):193-214. https://doi.org/10.1183/09059180.00001814
61. Funke-Chambour M, Azzola A, Adler D, Barazzone-Argiroffo C, Benden C, Boehler A, et al. Idiopathic Pulmonary Fibrosis in Switzerland: Diagnosis and Treatment. Respiration. 2017;93(5):363-378. https://doi.org/10.1159/000464332
62. Magnet FS, Schwarz SB, Callegari J, Criée CP, Storre JH, Windisch W. Long-Term Oxygen Therapy: Comparison of the German and British Guidelines. Respiration. 2017;93(4):253-263. https://doi.org/10.1159/000455879
63. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. https://doi.org/10.1183/13993003.01913-2018
64. Ulrich S, Saxer S, Hasler ED, Schwarz EI, Schneider SR, Furian M, et al. Effect of domiciliary oxygen therapy on exercise capacity and quality of life in patients with pulmonary arterial or chronic thromboembolic pulmonary hypertension: a randomised, placebo-controlled trial. Eur Respir J. 2019;54(2):1900276. https://doi.org/10.1183/13993003.002762019
65. Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164-172. https://doi.org/10.1161/CIRCULATIONAHA.109.898122
66. Trip P, Nossent EJ, de Man FS, van den Berk IA, Boonstra A, Groepenhoff H, et al. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J. 2013;42(6):1575-1585. https://doi.org/10.1183/09031936.00184412
67. Ulrich S, Hasler ED, Saxer S, Furian M, Müller-Mottet S, Keusch S, et al. Effect of breathing oxygen-enriched air on exercise performance in patients with precapillary pulmonary hypertension: randomized, sham-controlled cross-over trial. Eur Heart J. 2017;38(15):1159-1168. https://doi.org/10.1093/eurheartj/ehx099
68. Ulrich S, Keusch S, Hildenbrand FF, Lo Cascio C, Huber LC, Tanner FC, et al. Effect of nocturnal oxygen and acetazolamide on exercise performance in patients with pre-capillary pulmonary hypertension and sleep-disturbed breathing: randomized, double-blind, cross-over trial. Eur Heart J. 2015;36(10):615-623. https://doi.org/10.1093/eurheartj/eht540
69. Farber HW, Badesch DB, Benza RL, Elliott CG, Frantz RP, McGoon MD, Selej M, et al. Use of supplemental oxygen in patients with pulmonary arterial hypertension in REVEAL. J Heart Lung Transplant. 2018;37(8):948-955. https://doi.org/10.1016/j.healun.2018.03.010
70. Sandoval J, Aguirre JS, Pulido T, Martinez-Guerra ML, Santos E, Alvarado P, et al. Nocturnal oxygen therapy in patients with the Eisenmenger syndrome. Am J Respir Crit Care Med. 2001;164(9):1682-1687. https://doi.org/10.1164/ajrccm.164.9.2106076
71. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) [published correction appears in Eur Respir J. 2015 Dec;46(6):1855-6]. Eur Respir J. 2015;46(4):903-975. https://doi.org/10.1183/13993003.01032-2015
72. Oliveira EP, Medeiros Junior P. Palliative care in pulmonary medicine. J Bras Pneumol. 2020;46(3):e20190280. https://doi.org/10.36416/1806-3756/e20190280
73. Martins M, Campôa E, Ferreira M, Reis-Pina P. Autonomy and dyspnea in palliative care: A case report. Pulmonology. 2020;26(2):105-107. https://doi.org/10.1016/j.pulmoe.2019.05.005
74. Stanzani L, Reis-Pina P. Oxygen Therapy in Advanced Chronic Disease [Article in Portuguese]. Med Interna. 2020;27(2):186-187. doi: 10.24950/CE/74/20/2/2020 https://doi.org/10.24950/CE/74/20/2/2020
75. Campbell ML, Yarandi H, Dove-Medows E. Oxygen is nonbeneficial for most patients who are near death. J Pain Symptom Manage. 2013;45(3):517-523. https://doi.org/10.1016/j.jpainsymman.2012.02.012
76. Ambrosino N, Fracchia C. Strategies to relieve dyspnoea in patients with advanced chronic respiratory diseases. A narrative review. Pulmonology. 2019;25(5):289-298. https://doi.org/10.1016/j.pulmoe.2019.04.002
77. Gillon S, Clifton IJ. Breathlessness in palliative care: a practical guide. Br J Hosp Med (Lond). 2019;80(2):72-77. https://doi.org/10.12968/hmed.2019.80.2.72
78. Pan CX, Palathra BC, Leo-To WF. Management of Respiratory Symptoms in Those with Serious Illness. Med Clin North Am. 2020;104(3):455-470. https://doi.org/10.1016/j.mcna.2019.12.004
79. Verberkt CA, van den Beuken-van Everdingen MHJ, Schols JMGA, Datla S, Dirksen CD, Johnson MJ, et al. Respiratory adverse effects of opioids for breathlessness: a systematic review and meta-analysis. Eur Respir J. 2017;50(5):1701153. https://doi.org/10.1183/13993003.01153-2017
80. Ekström MP, Bornefalk-Hermansson A, Abernethy AP, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445. https://doi.org/10.1136/bmj.g445
81. Tobin MJ, Laghi F, Jubran A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am J Respir Crit Care Med. 2020;202(3):356-360. https://doi.org/10.1164/rccm.202006-2157CP
82. Busana M, Gasperetti A, Giosa L, Forleo GB, Schiavone M, Mitacchione G, et al. Prevalence and outcome of silent hypoxemia in COVID-19. Minerva Anestesiol. 2021;87(3):325-333. https://doi.org/10.23736/S0375-9393.21.15245-9
83. Fisher HK. Hypoxemia in COVID-19 patients: An hypothesis. Med Hypotheses. 2020;143:110022. https://doi.org/10.1016/j.mehy.2020.110022
84. Crimi C, Pierucci P, Renda T, Pisani L, Carlucci A. High-Flow Nasal Cannula and COVID-19: A Clinical Review. Respir Care. 2022;67(2):227-240. https://doi.org/10.4187/respcare.09056
85. Ospina-Tascón GA, Calderón-Tapia LE, García AF, Zarama V, Gómez-Álvarez F, Álvarez-Saa T, et al. Effect of High-Flow Oxygen Therapy vs Conventional Oxygen Therapy on Invasive Mechanical Ventilation and Clinical Recovery in Patients With Severe COVID-19: A Randomized Clinical Trial [published correction appears in JAMA. 2022 Mar 15;327(11):1093]. JAMA. 2021;326(21):2161-2171. https://doi.org/10.1001/jama.2021.20714
86. Loerinc LB, Scheel AM, Evans ST, Shabto JM, O’Keefe GA, O’Keefe JB. Discharge characteristics and care transitions of hospitalized patients with COVID-19. Healthc (Amst). 2021;9(1):100512. https://doi.org/10.1016/j.hjdsi.2020.100512
87. Ray A, Chaudhry R, Ray S, Mitra S, Pradhan S, Sunder A, et al. Prolonged Oxygen Therapy Post COVID-19 Infection: Factors Leading to the Risk of Poor Outcome. Cureus. 2021;13(2):e13357. https://doi.org/10.7759/cureus.13357
88. Spruit MA, Holland AE, Singh SJ, Tonia T, Wilson KC, Troosters T. COVID-19: Interim Guidance on Rehabilitation in the Hospital and Post-Hospital Phase from a European Respiratory Society and American Thoracic Society-coordinated International Task Force. Eur Respir J. 2020;56(6):2002197. https://doi.org/10.1183/13993003.02197-2020
89. Hayes D Jr, Wilson KC, Krivchenia K, Hawkins SMM, Balfour-Lynn IM, Gozal D, et al. Home Oxygen Therapy for Children. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2019;199(3):e5-e23. https://doi.org/10.1164/rccm.201812-2276ST
90. Krivchenia K, Hawkins SM, Iyer NP, Hayes D Jr, Deterding RR, Ruminjo J, et al. Clinical Practice Guideline Summary for Clinicians: Home Oxygen Therapy for Children. Ann Am Thorac Soc. 2019;16(7):781-785. https://doi.org/10.1513/AnnalsATS.201902-136CME
91. Rahimi S. New guidelines for home oxygen therapy in children. Lancet Respir Med. 2019;7(4):301-302. https://doi.org/10.1016/S2213-2600(19)30076-1
92. Adde FV, Alvarez AE, Barbisan BN, Guimarães BR. Recommendations for long-term home oxygen therapy in children and adolescents. J Pediatr (Rio J). 2013;89(1):6-17. https://doi.org/10.1016/j.jped.2013.02.003
93. Balfour-Lynn IM, Field DJ, Gringras P, Hicks B, Jardine E, Jones RC, et al. BTS guidelines for home oxygen in children. Thorax. 2009;64 Suppl 2:ii1-ii26. https://doi.org/10.1136/thx.2009.116020
94. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med. 2019;200(6):751-759. https://doi.org/10.1164/rccm.201812-2348OC
95. Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J Pediatr. 2018;197:300-308. https://doi.org/10.1016/j.jpeds.2018.01.043
96. Hennelly M, Greenberg RG, Aleem S. An Update on the Prevention and Management of Bronchopulmonary Dysplasia. Pediatric Health Med Ther. 2021;12:405-419. https://doi.org/10.2147/PHMT.S287693
97. Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. BMJ. 2021;375:n1974. https://doi.org/10.1136/bmj.n1974
98. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. https://doi.org/10.1001/jama.2015.10244
99. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105(2):295-310. https://doi.org/10.1542/peds.105.2.295
100. Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med. 2003;349(10):959-967. https://doi.org/10.1056/NEJMoa023080
101. BOOST II United Kingdom Collaborative Group; BOOST II Australia Collaborative Group; BOOST II New Zealand Collaborative Group, Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368(22):2094-2104. https://doi.org/10.1056/NEJMoa1302298
102. Cummings JJ, Polin RA; COMMITTEE ON FETUS AND NEWBORN. Oxygen Targeting in Extremely Low Birth Weight Infants [published correction appears in Pediatrics. 2016 Dec;138(6):]. Pediatrics. 2016;138(2):e20161576. https://doi.org/10.1542/peds.2016-1576
103. Duijts L, van Meel ER, Moschino L, Baraldi E, Barnhoorn M, Bramer WM, et al. European Respiratory Society guideline on long-term management of children with bronchopulmonary dysplasia. Eur Respir J. 2020;55(1):1900788. https://doi.org/10.1183/13993003.00788-2019
104. Athanazio RA, Silva Filho LVRF, Vergara AA, Ribeiro AF, Riedi CA, Procianoy EDFA, et al. Brazilian guidelines for the diagnosis and treatment of cystic fibrosis. J Bras Pneumol. 2017;43(3):219-245. https://doi.org/10.1590/s1806-37562017000000065
105. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479-504. https://doi.org/10.5664/jcsm.6506
106. Beaudin AE, Waltz X, Hanly PJ, Poulin MJ. Impact of obstructive sleep apnoea and intermittent hypoxia on cardiovascular and cerebrovascular regulation. Exp Physiol. 2017;102(7):743-763. https://doi.org/10.1113/EP086051
107. Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2019;15(2):335-343. https://doi.org/10.5664/jcsm.7640
108. Zeineddine S, Rowley JA, Chowdhuri S. Oxygen Therapy in Sleep-Disordered Breathing. Chest. 2021;160(2):701-717. https://doi.org/10.1016/j.chest.2021.02.017
109. Mehta V, Vasu TS, Phillips B, Chung F. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2013;9(3):271-279. https://doi.org/10.5664/jcsm.2500
110. Gold AR, Schwartz AR, Bleecker ER, Smith PL. The effect of chronic nocturnal oxygen administration upon sleep apnea. Am Rev Respir Dis. 1986;134(5):925-929. https://doi.org/10.1164/arrd.1986.134.5.925
111. Sun X, Luo J, Wang Y. Comparing the effects of supplemental oxygen therapy and continuous positive airway pressure on patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. Sleep Breath. 2021;25(4):2231-2240. https://doi.org/10.1007/s11325-020-02245-4
112. Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171. https://doi.org/10.5664/jcsm.27133
113. Javaheri S, Ahmed M, Parker TJ, Brown CR. Effects of nasal O2 on sleep-related disordered breathing in ambulatory patients with stable heart failure. Sleep. 1999;22(8):1101-1106. https://doi.org/10.1093/sleep/22.8.1101
114. Staniforth AD, Kinnear WJ, Starling R, Hetmanski DJ, Cowley AJ. Effect of oxygen on sleep quality, cognitive function and sympathetic activity in patients with chronic heart failure and Cheyne-Stokes respiration. Eur Heart J. 1998;19(6):922-928. https://doi.org/10.1053/euhj.1997.0861
115. Chen C, Wen T, Liao W. Nocturnal supports for patients with central sleep apnea and heart failure: a systemic review and network meta-analysis of randomized controlled trials. Ann Transl Med. 2019;7(14):337. https://doi.org/10.21037/atm.2019.06.72
116. Schwarz EI, Scherff F, Haile SR, Steier J, Kohler M. Effect of Treatment of Central Sleep Apnea/Cheyne-Stokes Respiration on Left Ventricular Ejection Fraction in Heart Failure: A Network Meta-Analysis. J Clin Sleep Med. 2019;15(12):1817-1825. https://doi.org/10.5664/jcsm.8092
117. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med. 2015;373(12):1095-1105. https://doi.org/10.1056/NEJMoa1506459
118. Gold AR, Bleecker ER, Smith PL. A shift from central and mixed sleep apnea to obstructive sleep apnea resulting from low-flow oxygen. Am Rev Respir Dis. 1985;132(2):220-223.
119. Randerath W, Verbraecken J, Andreas S, Arzt M, Bloch KE, Brack T, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1):1600959. https://doi.org/10.1183/13993003.00959-2016
120. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325-331. https://doi.org/10.1164/rccm.200912-1869OC
121. Machado MC, Vollmer WM, Togeiro SM, Bilderback AL, Oliveira MV, Leitão FS, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J. 2010;35(1):132-137. https://doi.org/10.1183/09031936.00192008
122. Mokhlesi B, Masa JF, Brozek JL, Gurubhagavatula I, Murphy PB, Piper AJ, et al. Evaluation and Management of Obesity Hypoventilation Syndrome. An Official American Thoracic Society Clinical Practice Guideline [published correction appears in Am J Respir Crit Care Med. 2019 Nov 15;200(10):1326]. Am J Respir Crit Care Med. 2019;200(3):e6-e24. https://doi.org/10.1164/rccm.201905-1071ST
123. Masa JF, Pépin JL, Borel JC, Mokhlesi B, Murphy PB, Sánchez-Quiroga MÁ. Obesity hypoventilation syndrome. Eur Respir Rev. 2019;28(151):180097. https://doi.org/10.1183/16000617.0097-2018
124. O’Driscoll BR, Howard LS, Earis J, Mak V; British Thoracic Society Emergency Oxygen Guideline Group; BTS Emergency Oxygen Guideline Development Group. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(Suppl 1):ii1-ii90. https://doi.org/10.1136/thoraxjnl-2016-209729
125. Dilektasli AG, Porszasz J, Stringer WW, Casaburi R. Physiologic Effects of Oxygen Supplementation During Exercise in Chronic Obstructive Pulmonary Disease. Clin Chest Med. 2019;40(2):385-395. https://doi.org/10.1016/j.ccm.2019.02.004
126. Dipla K, Boutou AK, Markopoulou A, Pitsiou G, Papadopoulos S, Chatzikosti A, et al. Exertional Desaturation in Idiopathic Pulmonary Fibrosis: The Role of Oxygen Supplementation in Modifying Cerebral-Skeletal Muscle Oxygenation and Systemic Hemodynamics. Respiration. 2021;100(6):463-475. https://doi.org/10.1159/000514320
127. Marillier M, Bernard AC, Verges S, Moran-Mendoza O, O’Donnell DE, Neder JA. Oxygen supplementation during exercise improves leg muscle fatigue in chronic fibrotic interstitial lung disease. Thorax. 2021;76(7):672-680. https://doi.org/10.1136/thoraxjnl-2020-215135
128. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation [published correction appears in Am J Respir Crit Care Med. 2014 Jun 15;189(12):1570]. Am J Respir Crit Care Med. 2013;188(8):e13-e64. https://doi.org/10.1164/rccm.201309-1634ST
129. Jarosch I, Gloeckl R, Damm E, Schwedhelm AL, Buhrow D, Jerrentrup A, et al. Short-term Effects of Supplemental Oxygen on 6-Min Walk Test Outcomes in Patients With COPD: A Randomized, Placebo-Controlled, Single-blind, Crossover Trial. Chest. 2017;151(4):795-803. https://doi.org/10.1016/j.chest.2016.11.044
130. Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med. 2003;168(9):1034-1042. https://doi.org/10.1164/rccm.200212-1525OC
131. Neunhäuserer D, Steidle-Kloc E, Weiss G, Kaiser B, Niederseer D, Hartl S, et al. Supplemental Oxygen During High-Intensity Exercise Training in Nonhypoxemic Chronic Obstructive Pulmonary Disease. Am J Med. 2016;129(11):1185-1193.
132. Dyer F, Callaghan J, Cheema K, Bott J. Ambulatory oxygen improves the effectiveness of pulmonary rehabilitation in selected patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2012;9(2):83-91. https://doi.org/10.1177/1479972312438702
133. Alison JA, McKeough ZJ, Leung RWM, Holland AE, Hill K, Morris NR, et al. Oxygen compared to air during exercise training in COPD with exercise-induced desaturation. Eur Respir J. 2019;53(5):1802429. https://doi.org/10.1183/13993003.02429-2018
134. Langer D, Gosselink R. Why does oxygen supplementation during exercise training in COPD patients with exercise-induced desaturation not consistently improve exercise capacity?. Eur Respir J. 2019;54(5):1901586. https://doi.org/10.1183/13993003.01586-2019
135. Schneeberger T, Jarosch I, Leitl D, Gloeckl R, Hitzl W, Dennis CJ, et al. Automatic oxygen titration versus constant oxygen flow rates during walking in COPD: a randomised controlled, double-blind, crossover trial [published online ahead of print, 2021 Oct 16]. Thorax. 2021;thoraxjnl-2020-216509. https://doi.org/10.1136/thoraxjnl-2020-216509
136. Vitacca M, Paneroni M, Zampogna E, Visca D, Carlucci A, Cirio S, et al. High-Flow Oxygen Therapy During Exercise Training in Patients With Chronic Obstructive Pulmonary Disease and Chronic Hypoxemia: A Multicenter Randomized Controlled Trial. Phys Ther. 2020;100(8):1249-1259. https://doi.org/10.1093/ptj/pzaa076
137. Liu Y, Gong F. Determination of whether supplemental oxygen therapy is beneficial during exercise training in patients with COPD: A systematic review and meta-analysis. Exp Ther Med. 2019;18(5):4081-4089. https://doi.org/10.3892/etm.2019.8026
138. Arizono S, Furukawa T, Taniguchi H, Sakamoto K, Kimura T, Kataoka K, et al. Supplemental oxygen improves exercise capacity in IPF patients with exertional desaturation. Respirology. 2020;25(11):1152-1159. https://doi.org/10.1111/resp.13829
139. Dowman LM, McDonald CF, Bozinovski S, Vlahos R, Gillies R, Pouniotis D, et al. Greater endurance capacity and improved dyspnoea with acute oxygen supplementation in idiopathic pulmonary fibrosis patients without resting hypoxaemia. Respirology. 2017;22(5):957-964. https://doi.org/10.1111/resp.13002
140. Nishiyama O, Miyajima H, Fukai Y, Yamazaki R, Satoh R, Yamagata T, et al. Effect of ambulatory oxygen on exertional dyspnea in IPF patients without resting hypoxemia. Respir Med. 2013;107(8):1241-1246. https://doi.org/10.1016/j.rmed.2013.05.015
141. Edvardsen A, Jarosch I, Grongstad A, Wiegand L, Gloeckl R, Kenn K, et al. A randomized cross-over trial on the direct effects of oxygen supplementation therapy using different devices on cycle endurance in hypoxemic patients with Interstitial Lung Disease. PLoS One. 2018;13(12):e0209069. https://doi.org/10.1371/journal.pone.0209069
142. Schaeffer MR, Ryerson CJ, Ramsook AH, Molgat-Seon Y, Wilkie SS, Dhillon SS, et al. Effects of hyperoxia on dyspnoea and exercise endurance in fibrotic interstitial lung disease. Eur Respir J. 2017;49(5):1602494. https://doi.org/10.1183/13993003.02494-2016
143. Grünig E, Eichstaedt C, Barberà J-A, Benjamin N, Blanco I, Bossone E, et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur Respir J. 2019;53(2):1800332. https://doi.org/10.1183/13993003.00332-2018
144. Marra AM, Egenlauf B, Bossone E, Eichstaedt C, Grünig E, Ehlken N. Principles of rehabilitation and reactivation: pulmonary hypertension. Respiration. 2015;89(4):265-273. https://doi.org/10.1159/000371855
145. Grünig E, Lichtblau M, Ehlken N, Ghofrani HA, Reichenberger F, Staehler G, et al. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J. 2012;40(1):84-92. https://doi.org/10.1183/09031936.00123711
146. Ahmedzai S, Balfour-Lynn IM, Bewick T, Buchdahl R, Coker RK, Cummin AR, et al. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax. 2011;66 Suppl 1:i1-i30. https://doi.org/10.1136/thoraxjnl-2011-200295
147. Bellinghausen AL, Mandel J. Assessing Patients for Air Travel. Chest. 2021;159(5):1961-1967. https://doi.org/10.1016/j.chest.2020.11.002
148. Ergan B, Akgun M, Pacilli AMG, Nava S. Should I stay or should I go? COPD and air travel. Eur Respir Rev. 2018;27(148):180030. https://doi.org/10.1183/16000617.0030-2018
149. Gong H Jr, Tashkin DP, Lee EY, Simmons MS. Hypoxia-altitude simulation test. Evaluation of patients with chronic airway obstruction. Am Rev Respir Dis. 1984;130(6):980-986.
150. Dillard TA, Moores LK, Bilello KL, Phillips YY. The preflight evaluation. A comparison of the hypoxia inhalation test with hypobaric exposure. Chest. 1995;107(2):352-357. https://doi.org/10.1378/chest.107.2.352
151. Sponholz Araújo J. Viagens aéreas em portadores de doença pulmonar avançada. In: Augusto V. Manual de Assistência Domiciliar em Doença Pulmonar Avançada. São Paulo: Grupo Editorial Nacional; 2013. p. 246-265.
152. Stoller J. Evaluation of patient for supplemental oxygen during air travel. Barnes PJ, Diffenbach P, editors. UpToDate. Waltham, MA: UpToDate Inc. [cited 2022 Mar 10]. Available from: https://www.uptodate.com/contents/evaluation-of-patients-for-supplemental-oxygen-during-air-travel
153. Orritt R, Powell P, Saraiva I. Why is medical oxygen a challenge for people travelling by air?. Breathe (Sheff). 2019;15(3):182-189. https://doi.org/10.1183/20734735.0202-2019
154. Agência Nacional de Aviação Civil (ANAC) [homepage on the Internet]. Brasília: ANAC; [updated 2021 Apr 15; cited 2022 Mar 1]. Instrução suplementar IS no. 119-007. Revisão A. Concentradores portáteis de oxigênio. Available from: https://www.anac.gov.br/assuntos/legislacao/legislacao-1/iac-e-is/is/is-119-007
155. Lacasse Y, Sériès F, Corbeil F, Baltzan M, Paradis B, Simão P, et al. Randomized Trial of Nocturnal Oxygen in Chronic Obstructive Pulmonary Disease. N Engl J Med. 2020;383(12):1129-1138. https://doi.org/10.1056/NEJMoa2013219
156. Global Initiative for Chronic Obstructive Lung Disease [homepage on the Internet]. Bethesda: GOLD [cited 2022 Mar 1]. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2022 report. Available from: https://goldcopd.org
157. Hardavella G, Karampinis I, Frille A, Sreter K, Rousalova I. Oxygen devices and delivery systems. Breathe (Sheff). 2019;15(3):e108-e116. https://doi.org/10.1183/20734735.0204-2019
158. Chest Foundation [homepage on the Internet]. Glenview (IL): the Foundation; [updated 2021 Nov 19; cited 2022 Mar 1]. Oxygen therapy (Last updated 19/11/2021). Available from: https://foundation.chestnet.org/lung-health-a-z/oxygen-therapy/
159. American Association for Respiratory Care [homepage on the Internet]. Irving (TX): the Association; [cited 2022 Mar 1]. A guide to portable concentrators. Available from: https://www.aarc.org/education/online-courses/a-guide-to-portable-oxygen-concentrators/
160. Tanni SE, Vale SA, Lopes PS, Guiotoko MM, Godoy I, Godoy I. Influence of the oxygen delivery system on the quality of life of patients with chronic hypoxemia. J Bras Pneumol 2007;33(2):161-167. https://doi.org/10.1590/S1806-37132007000200010
161. Schwartz MD Christopher KL, Schwartz EK. Transtracheal oxygen therapy. Stoller JK, Colt HG, Diffenbach P, editors. UpToDate. Waltham, MA: UpToDate Inc. [cited 2022 Mar 10]. Available from: UpTodate 2021. Available from: https://www.uptodate.com/contents/transtracheal-oxygen-therapy/print
162. Aguiar C, Davdson J, Carvalho A, Iamonti V, Cortopassi F, Nascimento O, et al. Tubing length for long-term oxygen therapy. Respir Care. 2015;60(2):179-182 https://doi.org/10.4187/respcare.03454