ABSTRACT
Objective: To identify microorganisms in sputum samples of patients with stable non-cystic fibrosis bronchiectasis and to determine risk factors related to the isolation of Pseudomonas aeruginosa (PA) in those patients. Methods: Consecutive patients were recruited from a tertiary hospital outpatient clinic in the city of Fortaleza, Brazil. The patients were submitted to spirometry, six-minute walk test, HRCT, and sputum collection. Data on serum fibrinogen levels, disease severity, sputum color, and history of azithromycin treatment were collected. Results: The study included 112 patients, and females predominated (68%). The mean age was 51.6 ± 17.4 years. Most patients presented with mild-to-moderate disease (83%). The mean six-minute walk distance was 468.8 ± 87.9 m. Mean FEV1 and FVC, in % of predicted values, were 60.4 ± 21.8% and 69.9 ± 18.5%, respectively. The mean serum fibrinogen level was 396.1 ± 76.3 mg/dL. PA was isolated in 47 patients, other potentially pathogenic microorganisms (PPMs) were isolated in 31 patients, and non-PPMs were isolated in 34 patients. Purulent sputum was identified in 77 patients (68%). The patients with PA, when compared with those without it, presented with more severe disease, higher serum fibrinogen levels, and lower FVC%. In addition, purulent sputum and long-term azithromycin treatment were more common in those with PA. The multivariate regression analysis showed that the independent factors associated with PA were serum fibrinogen level > 400 mg/dL (OR = 3.0; 95% CI: 1.1-7.7) and purulent sputum (OR = 4.3; 95% CI: 1.6-11.3). Conclusions: In our sample, the prevalence of PA in sputum was 42%. Sputum color and inflammatory markers were able to predict the isolation of PA, emphasizing the importance of routine sputum monitoring.
Keywords:
Bronchiectasis; Pseudomonas aeruginosa; Sputum/microbiology.
RESUMO
Objetivo: Identificar microrganismos em amostras de escarro de pacientes com bronquiectasia não fibrocística estável e determinar os fatores de risco relacionados com o isolamento de Pseudomonas aeruginosa (PA) nesses pacientes. Métodos: Pacientes consecutivos foram recrutados em um ambulatório de um hospital terciário em Fortaleza (CE). Os pacientes foram submetidos a espirometria, teste de caminhada de seis minutos, TCAR e coleta de escarro. Foram coletados dados referentes ao fibrinogênio sérico, gravidade da doença, cor do escarro e histórico de tratamento com azitromicina. Resultados: O estudo incluiu 112 pacientes, com predomínio do sexo feminino (68%). A média de idade foi de 51,6 ± 17,4 anos. A maioria dos pacientes apresentou doença leve a moderada (83%). A média da distância percorrida no teste de caminhada de seis minutos foi de 468,8 ± 87,9 m. A média do VEF1 em % do previsto foi de 60,4 ± 21,8%, e a da CVF em % do previsto foi de 69,9 ± 18,5%. A média do fibrinogênio sérico foi de 396,1 ± 76,3 mg/dL. PA foi isolada em 47 pacientes; outros microrganismos potencialmente patogênicos (MPP) foram isolados em 31; não MPP foram isolados em 34. Escarro purulento foi identificado em 77 pacientes (68%). Os pacientes com PA, em comparação com aqueles sem, apresentaram doença mais grave, fibrinogênio sérico mais elevado e menor CVF%. Além disso, escarro purulento e tratamento prolongado com azitromicina foram mais comuns naqueles com PA. A análise de regressão multivariada mostrou que os fatores independentes relacionados com PA foram fibrinogênio sérico > 400 mg/dL (OR = 3,0; IC95%: 1,1-7,7) e escarro purulento (OR = 4,3; IC95%: 1,6-11,3). Conclusões: Em nossa amostra, a prevalência de PA no escarro foi de 42%. A cor do escarro e os marcadores inflamatórios foram capazes de prever o isolamento de PA, o que enfatiza a importância do monitoramento rotineiro do escarro.
Palavras-chave:
Bronquiectasia; Pseudomonas aeruginosa; Escarro/microbiologia.
INTRODUCTION Bronchiectasis is a growing health problem worldwide. The disease prevalence has increased by more than 40% in Europe and the USA in the past 10 years.(1) Non-cystic fibrosis bronchiectasis is characterized by non-reversible bronchial dilatation, usually accompanied by cough, sputum production, and recurrent respiratory infections.(2)
Chronic bacterial infections are often present in patients with bronchiectasis, contributing to the maintenance of the vicious circle of inflammation and progressive destruction of airways. Systemic inflammation is crucial for disease progression, and that can be associated with more adverse events and worse outcomes. There are various inflammatory markers that can be used for evaluation of disease progression, including interleukins, TNF-α, C-reactive protein (CRP), and fibrinogen.(3) Inflammation is associated with airway bacterial infection and may be responsible for airway destruction and loss of lung function. Haemophilus influenzae and Pseudomonas aeruginosa have been the most common potentially pathogenic bacteria in bronchiectasis.(3,4)
Murray et al.(5) developed a quick and easy qualitative method to identify sputum color in patients with stable bronchiectasis. The sputum color chart uses photographs of sputum from patients with bronchiectasis, providing accurate representation of three major color grades, and showed good interobserver reliability between the doctor and the patient. Bacterial infection causes a pronounced increase in inflammatory markers that might be reflected by sputum purulence. This characteristic can be explored by clinicians using a sputum color chart.
Effects of P. aeruginosa on airway destruction might be indirectly reflected by lung function impairment.(6) Guan et al.(7) reported that a group of 144 patients with bronchiectasis and isolates of or infection with P. aeruginosa had poorer spirometry results. A recent study involving 186 patients followed at a bronchiectasis tertiary referral center in Portugal reported that patients with chronic bacterial infection had worse lung function when compared with those without that type of infection.(8)
Most studies on this topic have addressed the diversity of isolates of potentially pathogenic microorganisms (PPMs).(9,10) In patients with bronchiectasis, the few available data are mainly based on P. aeruginosa infection.(11) There is insufficient knowledge on P. aeruginosa infection, its associations, and useful clinical methods to distinguish P. aeruginosa from other PPMs.
Because of the importance of identifying bronchiectasis patients who are potentially infected with P. aeruginosa, new severity scores have been developed, such as FACED—an acronym for FEV1, Age, Chronic colonization with P. aeruginosa, Extent (of CT findings), and Dyspnea.(12)
Based on the hypothesis that patients infected with P. aeruginosa are a distinct group of patients within the group of patients with non-cystic fibrosis bronchiectasis, the objective of the present study was to assess the prevalence of P. aeruginosa isolation/infection in outpatients with bronchiectasis. Moreover, we expected that positive results could be associated with factors such as inflammatory markers and sputum color, as well as with clinical, radiological, and lung function parameters.
METHODS This was a cross-sectional study involving a group of adult patients with non-cystic fibrosis bronchiectasis who were consecutively selected from an outpatient clinic of a tertiary hospital between March of 2018 and October of 2019. Bronchiectasis was diagnosed on the basis of chest HRCT performed within the previous 12 months. Eligible patients had to remain exacerbation free for four weeks. Exacerbation was defined as the presence or worsening of three or more of the following key symptoms for at least 48 h: cough; high sputum volume/consistency; purulent sputum; breathlessness; exercise intolerance; fatigue; malaise; and hemoptysis.(2) The study protocol was approved by local research ethics committee (Protocol no. 1.844.662). All participants gave written informed consent.
We collected data on demographics; history of childhood respiratory infections (pertussis, pneumonia, and measles); history of pulmonary tuberculosis; diagnosed asthma, COPD, connective tissue disorders, and immune deficiencies; smoking status; history of long-term use of azithromycin; and treatment at the time of the last evaluation in a clinically stable phase. The participants were assessed regarding perception of dyspnea (modified Medical Research Council dyspnea score), sputum purulence/color (sputum color chart),(5) severity of bronchiectasis (FACED score),(12) and lung function (spirometry). Clinical assessment was performed by the attending doctor. Serum fibrinogen levels were also measured and compiled.
Samples of spontaneous sputum were obtained from all of the patients in the morning of their clinical visit. Gram-stained smears of the samples showing ≥ 25 leukocytes/field and ≤ 10 epithelial cells/field (magnification, ×100) were considered valid sputum samples and processed for qualitative culture for bacteria (including AFB) and fungi. All microbiological samples were plated on blood agar, chocolate agar, Wilkins-Chalgren agar, Löwenstein-Jensen medium, and Sabouraud agar. In addition, the samples were smeared for Ziehl-Neelsen staining. The cultures were evaluated for growth after 48 h. Negative bacterial cultures were discarded after 5 days, negative fungal cultures were discarded after four weeks, and Löwenstein-Jensen cultures were discarded after six weeks. Bacterial/fungal load (×105 CFU/mL) was calculated when a PPM was isolated. On the basis of culture results, the patients were divided into PA group (P. aeruginosa), PPM group (other than P. aeruginosa), and non-PPM group. The sputum color chart was shown to the patients so that they could identify one of the three typical color grades: clear (mucoid), pale yellow/pale green (mucopurulent), and dark yellow/dark green (purulent).(5)
HRCT scans were assessed for the number of lobes involved (the lingula was considered a separate lobe) and the most common type of bronchial dilatation (cylindrical, varicose, or cystic).(13)
Spirometry was performed using an electronic spirometer (WinDX; (Creative BioMedics Inc., San Clemente, CA, USA) in accordance with the American Thoracic Society/European Respiratory Society guidelines,(14) and FEV1 and FVC results were collected and analyzed.
Exercise capacity was evaluated using the six-minute walk test, which measures the distance that a participant can walk on a flat 30-m corridor in six minutes.(15) The patients, under direct supervision of one of the investigators, were asked to walk as fast as possible from one end of the corridor to the other, as many times as possible, within the established time. All patients performed two tests, with a minimum time interval of 30 min, and the best result was recorded.
The FACED score has been used as a tool to assess the severity of bronchiectasis.(12) As previously mentioned, the score incorporates five dichotomous variables, and the scores of each variable are summed up to provide the total score, which can range from 0 to 7 points. The total score classifies bronchiectasis into three levels of severity: mild (0-2 points), moderate (3-4 points), and severe (5-7 points). The FACED score has been validated for use in Brazil.(16)
Sample size A previous study showed that the prevalences of P. aeruginosa and other microorganisms in patients with bronchiectasis were 15% and 40%, respectively. (17) For the purpose of multivariate logistic regression analysis, the dependent variable was dichotomized as PA group or non-PA group (i.e., PPM + non-PPM groups), assuming that the rates of these groups were 15% and 40%, respectively. Defining α < 0.05 and β < 0.20, at least 57 participants were required for one arm.
Statistical analysis Categorical variables were described as absolute and relative frequencies, whereas continuous variables were described as mean and standard deviation, when appropriate.
One-way ANOVA was used in order to compare the means of the three groups individually, followed by a post hoc analysis with Bonferroni correction to clarify the differences between the pairs of groups (PA vs. PPM; PA vs. non-PPM; and PPM vs. non-PPM). For comparison of proportions, the chi-square test with post hoc analysis for pairwise comparisons was used with Bonferroni-adjusted p value.(18) As previously mentioned, the dependent variable was dichotomized as PA group or non-PA group. The independent factors selected for the multivariate analysis were those considered to be clinically relevant or potential confounders for the identification of PA isolates: sex (female); FVC (< 80% of the predicted value); sputum color(5) (purulent); serum fibrinogen level (> 400 mg/dL); and FACED total score(12) (≥ 5). Multicollinearity was assessed using the variance inflation factor (VIF); a VIF < 2.5 was regarded as an exclusion of any significant interaction.(19) The results were reported as OR and 95% CI. The significance level was set at p < 0.05. All statistical analyses were performed with the IBM SPSS Statistics software package, version 21.0 (IBM Corporation, Armonk, NY, USA).
RESULTS The flow chart of patient recruitment is shown in Figure 1. A total of 122 consecutive patients with non-cystic fibrosis bronchiectasis were initially included in the study. Of those, 10 did not meet the inclusion criteria and were excluded. Therefore, the total sample comprised 112 patients.
Table 1 shows that 77 patients (68%) were female. The mean age was 51.7 ± 17.4 years. According to the FACED score, 83% of the patients presented with mild-to-moderate disease. Mucopurulent/purulent sputum predominated (n = 77; 68%). Of those 77 patients with mucopurulent/purulent sputum, 70 (62% of the total sample) were submitted to long-term azithromycin treatment (500 mg, three times/week).
The etiology of bronchiectasis was determined by means of the review of clinical medical records. An underlying etiology was identified in 65% of the patients. In 35% of the patients no cause was established (classified as idiopathic bronchiectasis). The remaining etiologies were described as post-tuberculosis bronchiectasis, in 30%; post-infection bronchiectasis, in 5%; Kartagener syndrome, in 8%; and other etiologies, in 22%.
The microorganisms identified in the sputum of the patients are detailed in Table 2, whereas Table 3 shows the comparison of the selected variables between the groups (PA, PPM, and non-PPM). Significant differences were found regarding the following variables: serum fibrinogen levels, which were higher in the PA group when compared with the PPM group (425.4 ± 78.3 mg/dL vs. 380.5 ± 72.2 mg/dL; p = 0.04) and the non-PPM group (425.4 ± 78.3 mg/dL vs. 357.4 ± 75.5 mg/dL; p = 0.001); FVC in % of predicted values, which was lower in the PA group when compared with the PPM group (64.3% ± 16.5% vs. 75.9% ± 14.7%; p = 0.02); proportion of patients with purulent sputum, which was higher in the PA group when compared with the PPM (66.0% vs. 32.3%; p = 0.003) and non-PPM groups (66.0% vs. 14.7%; p < 0.001); severe bronchiectasis, which was higher in the PA group when compared with the non-PPM group (29.8% vs. 2.9%; p = 0.002); and long-term azithromycin treatment, which was more common in the PA group when compared with the non-PPM group (80.9% vs. 41.2%; p < 0.001).
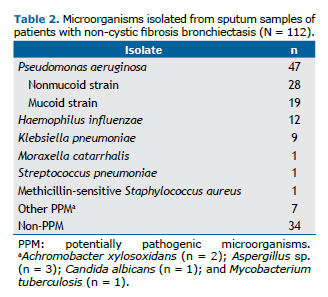
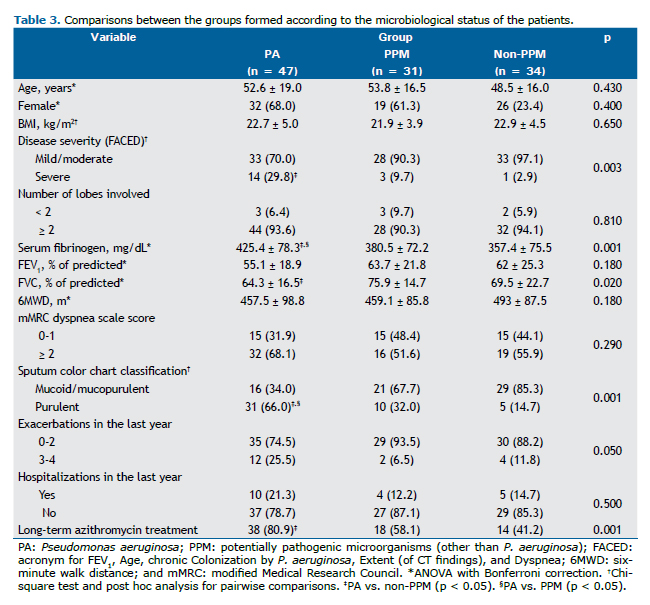
The multivariate logistic regression analysis was performed to determine the factors associated with the P. aeruginosa isolates (Table 4). Independent variables were gender, FACED score, serum fibrinogen level, FVC%, and sputum color classification. All of the factors had a VIF < 2.0. The independent factors associated with the isolation of P. aeruginosa were fibrinogen > 400 mg/dL (OR = 3.00; 95% CI: 1.10-7.77) and purulent sputum (OR = 4.33; 95% CI: 1.60-11.38).
DISCUSSION The present cross-sectional study showed that, in our sample of patients with steady-state bronchiectasis, 47 (42%) harbored P. aeruginosa in the airways. The rate of P. aeruginosa isolates was significantly higher than was that of H. influenzae, corroborating the findings in a study by Guan et al.(7) Multivariate logistic regression analysis identified that high levels of serum fibrinogen and purulent sputum were associated with isolation of P. aeruginosa. We would like to emphasize that the use of the sputum color chart by Murray et al.(5) provided novel evidence about this rapid and practical way for clinicians to predict the presence of P. aeruginosa in the airways and distinguish it from other microbiological statuses. This useful tool indicates the severity of inflammation, airway destruction, and proteolytic enzyme activity/presence of neutrophilic airway disease, such as non-cystic fibrosis bronchiectasis, as well as COPD or asthma.(20-22)
The correlation of sputum color with positive cultures is not very clear, findings of positive and negative relationships having been described.(22-24) A recent meta-analysis(25) analyzing six studies on sputum staining and positive cultures in COPD patients showed that the isolation of bacteria in sputum is less likely to occur when sputum is classified as mucoid. More patients with purulent sputum presented with bacterial colonization than did patients with mucopurulent or mucoid sputum.
We found that the presence of a systemic inflammatory response (as evidenced by elevated circulating fibrinogen levels) was associated with the isolation of P. aeruginosa. Fibrinogen levels were higher in the PA group when compared with the PPM and non-PPM groups, which might explain the role of P. aeruginosa in systemic inflammation. Previous studies found that airways harboring P. aeruginosa showed significantly higher airway inflammation.(7,26-29)
Menéndez et al.(29) conducted a prospective observational study and found progressive increases in the levels of systemic proinflammatory cytokines and CRP in hospitalized patients with bronchiectasis from whom P. aeruginosa was isolated during acute and chronic phases of exacerbations. The level of systemic inflammation remained high after the acute phase. Jin et al.(30) found that systemic inflammatory markers, including CRP and fibrinogen, were significantly elevated in COPD patients with bronchiectasis. The use of other inflammatory markers is necessary to detect the severity of inflammation so that better treatment can be provided for patients with bronchiectasis. We decided to measure serum fibrinogen levels, because serum fibrinogen is a biomarker for which routine measurements are available in clinical practice.
Ergan Arsava & Cöplü,(31) studied 50 patients with stable bronchiectasis and found that fibrinogen and CRP levels were higher in those with airway colonization than in those without it. In a subgroup of patients colonized with P. aeruginosa, those levels were even higher than were those in their counterparts.
In our study, the presence of P. aeruginosa in the airways of patients was associated with reduced FVC. This could be explained by the effects of P. aeruginosa on inflammation and destruction of the airways. Studies have shown that when P. aeruginosa or H. influenzae dominates the microbiome of patients with bronchiectasis, their lung function is significantly reduced.(10,32)
The frequency of exacerbations and hospitalizations in the previous year in our sample of patients with P. aeruginosa was not statistically significant. This could be explained by the long-term use of azithromycin by these patients. A clinical trial conducted by Richardson et al.(32) demonstrated a significant reduction in the number of exacerbations in patients treated with erythromycin when compared with those treated with placebo. A meta-analysis of nine studies (530 patients) demonstrated that macrolide use reduced the number of patients with exacerbations and the number of exacerbations per patient.(33) The small number of hospitalizations was probably related to the small number of exacerbations in our study.
More severe disease, measured by the FACED score, was associated with the isolation of P. aeruginosa. This is expected because this tool incorporates colonization by P. aeruginosa in its metrics, emphasizing the importance of chronic infection in the severity of bronchiectasis.(12)
The number of lobes involved on CT scans was not found to be associated with the isolation of P. aeruginosa. Therefore, we cannot rule out or confirm that the presence of P. aeruginosa is a factor related to greater radiological structural damage in these patients.
The limitations of the present study include the following: i) the sample size was small, but we were able to identify variables with biological plausibility; ii) patients were recruited at a referral facility, making it difficult to extrapolate our results to other realities; iii) the follow-up period was short, and no molecular methods were used in order to understand the role that each microorganism plays in disease progression; and iv) the observational, cross-sectional design makes it difficult to establish temporal order and causal direction.
The present study is relevant because we might assume that a useful clinical method such as a sputum color chart(5) is able to predict airway infection with P. aeruginosa. Identifying sputum color in association with clinical manifestations of infection might be a useful strategy for clinicians to manage these patients while awaiting formal sputum microbiology results. In addition, the use of serum fibrinogen as a marker is a simple and reliable method to identify infected patients and should therefore be part of routine clinical practice. Larger multicenter longitudinal studies are needed to improve the characterization of other PPMs and their individual clinical impact.
AUTHOR CONTRIBUTIONS IL and AA: study design; data collection; literature search; and approval of the final version. MRF: study design; data collection; literature search; drafting of the manuscript; approval of the final version. FL: drafting of the manuscript; and approval of the final version. EDBP: study design; drafting of the manuscript; final revision; and approval of the final version.
REFERENCES 1. Henkle E, Chan B, Curtis JR, Aksamit TR, Daley CL, Winthrop KL. Characteristics and Health-care Utilization History of Patients With Bronchiectasis in US Medicare Enrollees With Prescription Drug Plans, 2006 to 2014. Chest. 2018;154(6):1311-1320. https://doi.org/10.1016/j.chest.2018.07.014
2. Pereira MC, Athanazio RA, Dalcin PTR, Figueiredo MRF, Gomes M, Freitas CG, et al. Brazilian consensus on non-cystic fibrosis bronchiectasis. J Bras Pneumol. 2019;45(4):e20190122. https://doi.org/10.1590/1806-3713/e20190122
3. O’Donnell AE. Bronchiectasis. Chest. 2008;134(4):815-823. https://doi.org/10.1378/chest.08-0776
4. Angrill J, Agustí C, de Celis R, Rañó A, Gonzalez J, Solé T, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57(1):15-19. https://doi.org/10.1136/thorax.57.1.15
5. Murray MP, Pentland JL, Turnbull K, MacQuarrie S, Hill AT. Sputum colour: a useful clinical tool in non-cystic fibrosis bronchiectasis. Eur Respir J. 2009;34(2):361-364. https://doi.org/10.1183/09031936.00163208
6. Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, Román-Sánchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest. 2007;132(5):1565-1572. https://doi.org/10.1378/chest.07-0490
7. Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Li HM, et al. Effect of airway Pseudomonas aeruginosa isolation and infection on steady-state bronchiectasis in Guangzhou, China. J Thorac Dis. 2015;7(4):625-636.
8. Amorim A, Meira L, Redondo M, Ribeiro M, Castro R, Rodrigues M, et al. Chronic Bacterial Infection Prevalence, Risk Factors, and Characteristics: A Bronchiectasis Population-Based Prospective Study. J Clin Med. 2019;8(3):315. https://doi.org/10.3390/jcm8030315
9. King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101(8):1633-1638. https://doi.org/10.1016/j.rmed.2007.03.009
10. Rogers GB, van der Gast CJ, Cuthbertson L, Thomson SK, Bruce KD, Martin ML, et al. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax. 2013;68(8):731-737. https://doi.org/10.1136/thoraxjnl-2012-203105
11. Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A Comprehensive Analysis of the Impact of Pseudomonas aeruginosa Colonization on Prognosis in Adult Bronchiectasis. Ann Am Thorac Soc. 2015;12(11):1602-1611. https://doi.org/10.1513/AnnalsATS.201506-333OC
12. de la Rosa Carrillo D, Athanazio R, Girón Moreno RM, Máiz Carro L, Olveira C, de Gracia J, et al. The annual prognostic ability of FACED and E-FACED scores to predict mortality in patients with bronchiectasis. ERJ Open Res. 2018;4(1):00139-2017. https://doi.org/10.1183/23120541.00139-2017
13. Martínez-García MÁ, de Gracia J, Vendrell Relat M, Girón RM, Máiz Carro L, de la Rosa Carrillo D, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J. 2014;43(5):1357-1367. https://doi.org/10.1183/09031936.00026313
14. Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70-e88. https://doi.org/10.1164/rccm.201908-1590ST
15. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test [published correction appears in Am J Respir Crit Care Med. 2016 May 15;193(10):1185]. Am J Respir Crit Care Med. 2002;166(1):111-117. https://doi.org/10.1164/ajrccm.166.1.at1102
16. Athanazio R, Pereira MC, Gramblicka G, Cavalcanti-Lundgren F, de Figueiredo MF, Arancibia F, et al. Latin America validation of FACED score in patients with bronchiectasis: an analysis of six cohorts. BMC Pulm Med. 2017;17(1):73. https://doi.org/10.1186/s12890-017-0417-3
17. Dhar R, Singh S, Talwar D, Mohan M, Tripathi SK, Swarnakar R, et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry [published correction appears in Lancet Glob Health. 2019 Dec;7(12):e1621]. Lancet Glob Health. 2019;7(9):e1269-e1279. https://doi.org/10.1016/S2214-109X(19)30445-0
18. MacDonald PL, Gardner RC. Type I Error Rate Comparisons of Post Hoc Procedures for I j Chi-Square Tables. Educ Psychol Meas. 2000;60(5):735-754. doi:10.1177/00131640021970871 https://doi.org/10.1177/00131640021970871
19. Allison PD, editor. Logistic Regression Using the SAS System: Theory and Application. New York: John Wiley & Sons; 2001
20. Miravitlles M, Kruesmann F, Haverstock D, Perroncel R, Choudhri SH, Arvis P. Sputum colour and bacteria in chronic bronchitis exacerbations: a pooled analysis. Eur Respir J. 2012;39(6):1354-1360. https://doi.org/10.1183/09031936.00042111
21. Pabreja K, Gibson P, Lochrin AJ, Wood L, Baines KJ, Simpson JL. Sputum colour can identify patients with neutrophilic inflammation in asthma. BMJ Open Respir Res. 2017;4(1):e000236. https://doi.org/10.1136/bmjresp-2017-000236
22. Goeminne PC, Vandooren J, Moelants EA, Decraene A, Rabaey E, Pauwels A, et al. The Sputum Colour Chart as a predictor of lung inflammation, proteolysis and damage in non-cystic fibrosis bronchiectasis: a case-control analysis. Respirology. 2014;19(2):203-210. https://doi.org/10.1111/resp.12219
23. Brusse-Keizer MG, Grotenhuis AJ, Kerstjens HA, Telgen MC, van der Palen J, Hendrix MG, et al. Relation of sputum colour to bacterial load in acute exacerbations of COPD. Respir Med. 2009;103(4):601-606. https://doi.org/10.1016/j.rmed.2008.10.012
24. Chalmers JD, Finch S. Sputum colour in non-CF bronchiectasis: the original neutrophil biomarker. Respirology. 2014;19(2):153-154. https://doi.org/10.1111/resp.12228
25. Chen K, Pleasants KA, Pleasants RA, Beiko T, Washburn RG, Yu Z, et al. A Systematic Review and Meta-Analysis of Sputum Purulence to Predict Bacterial Infection in COPD Exacerbations. COPD. 2020;17(3):311-317. https://doi.org/10.1080/15412555.2020.1766433
26. Hartl D, Griese M, Kappler M, Zissel G, Reinhardt D, Rebhan C, et al. Pulmonary T(H)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol. 2006;117(1):204-211. https://doi.org/10.1016/j.jaci.2005.09.023
27. Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2012;186(7):657-665. https://doi.org/10.1164/rccm.201203-0487OC
28. Aliberti S, Lonni S, Dore S, McDonnell MJ, Goeminne PC, Dimakou K, et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J. 2016;47(4):1113-1122. https://doi.org/10.1183/13993003.01899-2015
29. Menéndez R, Méndez R, Amara-Elori I, Reyes S, Montull B, Feced L, et al. Systemic Inflammation during and after Bronchiectasis Exacerbations: Impact of Pseudomonas aeruginosa. J Clin Med. 2020;9(8):2631. https://doi.org/10.3390/jcm9082631
30. Jin J, Yu W, Li S, Lu L, Liu X, Sun Y. Factors associated with bronchiectasis in patients with moderate-severe chronic obstructive pulmonary disease. Medicine (Baltimore). 2016;95(29):e4219. https://doi.org/10.1097/MD.0000000000004219
31. Ergan Arsava B, Cöplü L. Does airway colonization cause systemic inflammation in bronchiectasis?. Tuberk Toraks. 2011;59(4):340-347. https://doi.org/10.5578/tt.2934
32. Richardson H, Dicker AJ, Barclay H, Chalmers JD. The microbiome in bronchiectasis. Eur Respir Rev. 2019;28(153):190048. https://doi.org/10.1183/16000617.0048-2019
33. Wu Q, Shen W, Cheng H, Zhou X. Long-term macrolides for non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis. Respirology. 2014;19(3):321-329. https://doi.org/10.1111/resp.12233






