ABSTRACT
Objective: To estimate the prevalence of latent Mycobacterium tuberculosis infection (LTBI) in renal transplant recipients and to assess sociodemographic, behavioral, and clinical associations with positive tuberculin skin test (TST) results. Methods: This was a cross-sectional study of patients aged ≥ 18 years who underwent renal transplantation at the Renal Transplant Center of the Federal University of Minas Gerais Hospital das Clínicas, located in the city of Belo Horizonte, Brazil. We included renal transplant recipients who underwent the TST between January 2011 and July 2013. If the result of the first TST was negative, a second TST was administered. Bivariate and multivariate analyses using logistic regression were used to determine factors associated with positive TST results. Results: The sample included 216 patients. The prevalence of LTBI was 18.5%. In the multivariate analysis, history of contact with a tuberculosis case and preserved graft function (estimated glomerular filtration rate ≥ 60 mL/min/1.73 m2) were associated with positive TST results. TST induration increased by 5.8% from the first to the second test, which was considered significant (p = 0.012). Conclusions: The prevalence of LTBI was low in this sample of renal transplant recipients. The TST should be administered if renal graft function is preserved. A second TST should be administered if the first TST is negative.
Keywords:
Tuberculosis; Tuberculin test; Immunocompromised host.
RESUMO
Objetivo: Estimar a prevalência da infecção latente por Mycobacterium tuberculosis (ILTB) em transplantados renais e avaliar as associações sociodemográficas, comportamentais e clínicas com a prova tuberculínica (PT) positiva. Métodos: Estudo transversal, com pacientes com idade ≥ 18 anos, transplantados renais no Centro de Transplante Renal do Hospital das Clínicas da Universidade Federal de Minas Gerais, em Belo Horizonte (MG). Foram incluídos os pacientes submetidos a transplante renal que realizaram a PT no período entre janeiro de 2011 e julho de 2013. Quando o resultado da primeira PT foi negativo, uma segunda PT foi realizada. As análises bivariada e multivariada, por meio de regressão logística, foram utilizadas para determinar os fatores associados com PT positiva. Resultados: A amostra incluiu 216 pacientes. A taxa de prevalência para ILTB foi de 18.5%. Na análise multivariada, história de contato com caso de tuberculose e função do enxerto preservada (taxa de filtração glomerular estimada ≥ 60 ml/min/1,73 m2) foram associadas com PT positiva. O incremento da primeira PT para a segunda PT foi de 5,8%, considerado significante (p = 0,012). Conclusões: A prevalência da ILTB foi baixa nessa amostra de pacientes transplantados renais. A PT deve ser efetuada quando a função do enxerto renal estiver preservada. Uma segunda PT deve ser realizada quando a primeira PT for negativa.
Palavras-chave:
Tuberculose; Teste tuberculínico; Hospedeiro imunocomprometido.
INTRODUÇÃOSegundo o Registro Brasileiro de Transplantes, o número absoluto de transplantes renais de janeiro a dezembro de 2016 foi de 5.492 no Brasil, sendo 563 no estado de Minas Gerais (MG). Há 21.264 candidatos a transplante renal em lista de espera em todo Brasil, sendo 2.297 pacientes desse estado.(1)
A incidência da tuberculose nos transplantados renais comparada à da população geral é aproximadamente 20 a 74 vezes mais elevada (0,5-15% entre receptores de rim)(2) e oscila de acordo com a área geográfica (0,5% a 1% na América do Norte).(3)
Atualmente, os fármacos imunossupressores têm ações farmacológicas mais específicas e potentes para evitar a rejeição do enxerto, principalmente nos receptores de doadores falecidos com alto risco imunológico, que necessitam de terapia com anticorpos para a prevenção de rejeição humoral precoce.(4) No entanto, podem apresentar efeitos de toxicidade(5) e predispor o paciente a maiores riscos de infecções,(6) dentre elas, tuberculose e neoplasia.
A forma mais comum de infecção da tuberculose após o transplante é a reativação da infecção latente por Mycobacterium tuberculosis (ILTB). O desenvolvimento da doença é favorecido pela imunossupressão, e a maioria dos casos de tuberculose ocorre no primeiro ano após transplante.(2,6,7)
Na maioria dos países, a prova tuberculínica (PT) é utilizada para o diagnóstico da ILTB, apresentando sensibilidade de aproximadamente 70%, apesar de vários fatores que interferem no resultado, como farmacocinética dos imunossupressores, terapia de indução, terapia prévia para rejeição celular ou humoral, infecção por citomegalovírus (CMV), tempo de transplante, retransplante, estágio da doença renal crônica (DRC) após o transplante, diabetes mellitus (DM), entre outros.(8)
A PT para detecção da ILTB é relevante como teste propedêutico e, consequentemente, para a indicação da terapia de prevenção nos casos positivos, podendo contribuir com a diminuição da taxa de tuberculose nos indivíduos transplantados renais.(9,10) Entretanto, a PT não é rigorosamente realizada nos centros transplantadores do país.(11,12) Ressalta-se a escassa publicação de trabalhos sobre esse tema no Brasil.
Assim, o objetivo do presente estudo foi estimar a prevalência da ILTB em transplantados renais e avaliar as associações sociodemográficas, comportamentais e clínicas com resultados de PT positivos.
MÉTODOSTrata-se de um estudo transversal, conduzido no Centro de Transplante Renal do Hospital das Clínicas da Universidade Federal de MG (UFMG), localizado na cidade de Belo Horizonte (MG). Todos os transplantados renais foram avaliados para ILTB no período entre janeiro de 2011 e julho de 2013 por meio da PT. O estudo foi aprovado pelo Comitê de Ética em Pesquisa da UFMG, protocolo no. 132/10.
População do estudoPara o cálculo amostral, consideraram-se 324 pacientes potencialmente elegíveis do Ambulatório de Transplante Renal do hospital; admitindo-se um intervalo de confiança de 95%, erro de 5% e prevalência de 15% para ILTB (conforme estudo prévio),(6) a amostra deveria conter 160 pacientes. Foi acrescida uma taxa de provável recusa de 30%, sendo então, a amostra mínima calculada definida com 208 pacientes. Os critérios de inclusão foram: idade ≥ 18 anos e período mínimo de três meses após realização do transplante. Os critérios de exclusão foram: 1) história de tuberculose tratada antes ou após o transplante; 2) tratamento de prevenção com isoniazida antes do transplante; 3) perda do enxerto renal e retorno para terapia dialítica antes da realização da primeira PT (PT1) ou da segunda (PT2); 4) óbito; 5) não aderência à terapia imunossupressora; 6) comparecimento menor que duas consultas anuais ao ambulatório de transplante; ou 7) falta de assinatura do termo de consentimento (Figura 1).
 Triagem para ILTB
Triagem para ILTBPara a avaliação da ILTB foi realizada a PT com derivado proteico purificado (PPD RT23; Statens Serum Institut, Copenhague, Dinamarca). O PPD foi aplicado na dose de 0,1 ml, contendo duas unidades de tuberculina, utilizando-se a técnica intradérmica de Mantoux. O resultado da PT foi registrado em milímetros. O local da injeção foi na face anterior do antebraço e sua leitura realizada em 72-96 h. A PT1 foi realizada após o terceiro mês da realização do transplante renal e a PT2 a partir da terceira semana quando a PT1 fosse negativa, para avaliar a reativação da resposta imunológica. Todos os pacientes que apresentaram PT1 com valor ≥ 5 mm foram considerados positivos; aqueles com resultados negativos foram encaminhados para a PT2, sendo essa considerada positiva quando houve um incremento > 10 mm em relação a PT1. (13-15) A frequência acumulativa da ILTB também foi calculada (N = 216).
Variáveis e definiçõesAs variáveis investigadas foram: (i) variáveis sociodemográficas (gênero, idade, renda individual, moradia e história de contato com tuberculose); (ii) variáveis comportamentais (tabagismo, etilismo e situação conjugal); (iii) variáveis clínicas (marca vacinal da BCG, índice de massa corporal [IMC], DM, doença autoimune, hepatite B, hepatite C e neoplasias); (iv) variáveis relacionadas ao transplante (doador vivo/falecido, transplante duplo, retransplante, esquema imunossupressor, intervalo de tempo entre a cirurgia do transplante e realização do PT e função do enxerto renal [FER] pela taxa de filtração glomerular).
Os pacientes foram classificados como "ter renda individual" (empregado, aposentado ou afastado/seguro doença), ou "sem renda" (desempregado ou nunca trabalhou). O alcoolismo foi classificado de acordo com questionário Cut down, Annoyed, Guilty, and Eye-opener e incorporado à entrevista do paciente.(16) Os pacientes foram classificados como "fumantes" ou "não fumantes" (pessoas que nunca fumaram ou pessoas que abandonaram o hábito de fumar um ano antes do estudo).(17) Para avaliar a vacina BCG, foi observada a presença ou a ausência da cicatriz vacinal no braço direito. A idade dos receptores renais foi categorizada de acordo com a mediana de idade da população do estudo. O cálculo para o IMC seguiu as recomendações da Organização Mundial de Saúde.(18) Os pacientes foram categorizados como obesos (IMC > 30 kg/m2) ou não obesos (18,5 < IMC ≤ 29,9 kg/m2). O diagnóstico de DM foi categorizado de acordo com a classificação proposta pelas diretrizes da American Diabetes Association(19) e Sociedade Brasileira de Diabetes.(20) Para FER foi avaliada a estimated glomerular filtration rate (eGFR, taxa de filtração glomerular estimada) pela equação Modification of Diet in Renal Disease.(21) A função do enxerto renal foi categorizada como "função renal preservada" para valores de eGFR ≥ 60 ml/min/1,73 m2 ou como "função renal comprometida" se eGFR < 59 ml/min/1,73 m2.
Análise estatísticaFoi realizada a análise descritiva da população por meio de distribuições de frequências e medidas de tendência central e de dispersão para as características estudadas. O teste t de Student para amostras independentes foi utilizado para comparar diferenças de médias para as variáveis contínuas, e o teste do qui-quadrado de Pearson ou o teste exato de Fisher foi utilizado para a comparação de proporções das variáveis categóricas. Para todos os testes, o valor de p ≤ 0,05 foi considerado significante. A medida de associação utilizada na análise bivariada foi OR com IC95%.
As variáveis explicativas com valor p ≤ 0,20 na análise bivariada foram selecionadas para a análise multivariada pelo modelo da regressão logística. O nível de significância necessário para a inclusão no modelo final foi de 0,05, com ajuste para fatores de confusão. A adequação do modelo final foi verificada pelo teste de Hosmer-Lemeshow.
As informações coletadas foram digitadas em planilhas no Excel. O pacote estatístico IBM SPSS Statistics, versão 21.0 (IBM Corporation, Armonk, NY, EUA) foi utilizado para fazer as análises estatísticas, junto com recursos do programa R, versão 2.15.1 (The R Foundation for Statistical Computing, Viena, Áustria).
RESULTADOSAs características da população estudada (N = 216) e as causas da DRC estão demonstradas na Tabela 1. A idade no momento do teste variou de 18 a 75 anos, com mediana de 48 anos e média de 46,5 ± 12,3 anos. História de contato com tuberculose foi positiva em 38 pacientes (17,6%), negativa em 168 (77,8%), e desconhecida em 10 (4,6%). A obesidade estava presente em 23 pacientes (10,6%), e 54 pacientes (25%) eram diabéticos, dos quais 13 foram diagnosticados com DM tipo I antes do transplante e 41 com DM tipo II ou diabetes induzido por drogas após o transplante. Sete pacientes (3,2%) tiveram diagnóstico prévio de doença autoimune. Neoplasia depois do transplante, incluindo câncer de pele, esteve presente em 23 (10,6%) dos pacientes.
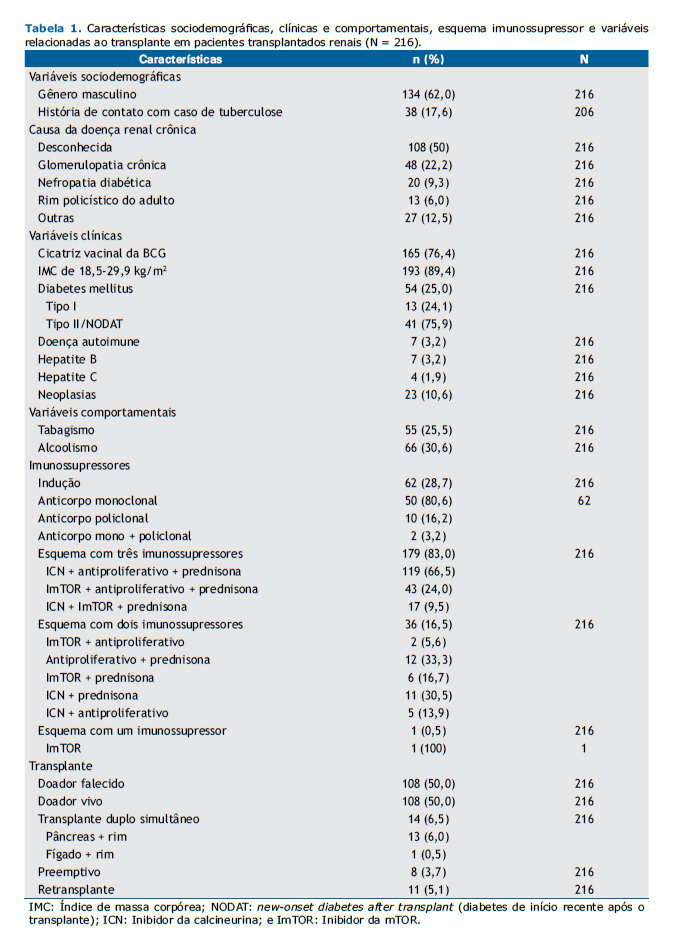
Dos 216 pacientes incluídos no estudo, 167 (77,3%) relataram ter renda por atividade laboral, aposentadoria ou benefício doença. Em relação ao local de residência, 152 (70,4%) moravam na região metropolitana de Belo Horizonte próximo ao centro transplantador, 63 (29,2%) moravam em outras regiões de MG, e 1 (0,4%) morava no estado do Amapá.
O intervalo de tempo entre data do transplante de rim e a realização da PT1 variou de 3,0 a 360,4 meses, com média de 86,8 ± 75,6 meses e mediana de 68,2 meses. O intervalo de tempo entre a data do transplante renal e a realização da PT2 variou de 3,5 a 376,1 meses, com média de 99,0 ± 78,3 meses e mediana de 79 meses.
A prevalência de ILTB foi de 18,5%, com 40 indivíduos mostrando positividade para a PT. A positividade foi detectada em 29 pacientes (13,4%) na PT1 e em 11 (5,1%) na PT2. O incremento da PT1 para PT2 foi de 5,8%, sendo esse significativo (p = 0,012).
A frequência acumulativa da ILTB na população estudada (inicial, PT1 e PT2) foi de 42,5%, pois, dos 216 pacientes incluídos no estudo, 40 já traziam o resultado positivo da PT (18,5%); dos 176 restantes, 29 apresentaram a PT1 positiva (16,5%), portanto restaram 147 pacientes para realizar a PT2, desses, 11 foram positivos (7,5%).
Na análise bivariada, considerando um valor de p ≤ 0,20, os fatores associados ao diagnóstico de ILTB foram ter história de contato com caso de tuberculose, alcoolismo, presença de cicatriz da BCG, eGFR ≥ 60 ml/min/1,73 m2, transplante duplo de órgãos e transplante preemptivo (transplante realizado antes do início do programa dialítico). No modelo final de regressão logística, estiveram associadas de forma estatisticamente significante (p ≤ 0,05) com diagnóstico de ILTB as seguintes variáveis: ter história de contato com caso de tuberculose, cicatriz da BCG e eGFR ≥ 60 ml/min/1,73 m2 (Tabela 2).
 DISCUSSÃO
DISCUSSÃOVários estudos mostram uma maior prevalência de tuberculose nos pacientes submetidos ao transplante renal em países de baixa, média e alta prevalência da doença, quando infectados pelo M. tuberculosis. (4,12,22) Assim, há necessidade de diagnosticar ILTB, e indicar terapia de prevenção é relevante para impedir o desenvolvimento da doença,(13) apesar de isso não ser rotineiramente realizado na prática clinica no Brasil. Apesar da indicação de incluir os pacientes candidatos a transplante para a realização da PT,(13) não há estudos que descrevam essa prática. No presente estudo, observou-se que a frequência da ILTB na nossa população foi alta (42,5%). Dessa forma, pelo que sabemos, esta é a primeira vez em que se avalia a ILTB e suas associações com características sociodemográficas, comportamentais e clínicas em transplantados renais em um centro de transplante no Brasil.
A realização da PT para detectar ILTB é preconizada em vários países na avaliação pré-transplante renal,(4) inclusive no Brasil, onde o interferon-gamma release assay (IGRA) não é validado em rotina.(13,14) Alguns fatores podem gerar resultados de PT falso-negativos, tais como DM, farmacocinética dos imunossupressores, indução e terapia prévia para rejeição humoral, infecção por CMV, entre outros.(10,13) Em 2015, a Organização Mundial da Saúde descreveu que IGRA ou PT podem ser usados para a identificação da ILTB, sendo fortemente recomendados, porém com baixo nível de evidência.(14)
A etiologia predominante da DRC antes do transplante foi indeterminada, porque a maioria dos pacientes do presente estudo não realizou biópsia renal para a confirmação histológica da DRC. Vale ressaltar que as glomerulopatias, no presente estudo, foram importantes, como já citado em outro trabalho.(7) Alcoolismo e tabagismo são fatores de risco para a ILTB e desenvolvimento da tuberculose.(23-26) Como, em nosso estudo, a maioria dos pacientes não ingeria álcool e não fumava, não houve associação estatística desses fatores (não foram fatores de risco) com a resposta positiva a PT.
Os pacientes submetidos ao transplante de órgãos são mais susceptíveis a infecções devido ao uso de drogas imunossupressoras. Porém, no nosso estudo, não foi identificada uma associação com o uso de imunossupressores. Assim, a melhor estratégia é avaliar a ILTB antes de se realizar o transplante do orgão. A Organização Mundial da Saúde recomenda aos países de alta ou média renda com baixa taxa de incidência de tuberculose (< 100 casos por 100.000 habitantes) testar e tratar a ILTB em pacientes que se preparam para transplante de órgãos ou transplante hematológico.(14)
Uso de tacrolimus e/ou micofenolato em receptores jovens, DM,(27) idade do receptor,(8) tempo de transplante,(7,12) hepatite C,(28) infecção por CMV, câncer e doenças autoimunes(8) são fatores descritos para a reativação e o desenvolvimento de tuberculose com gravidade e maior frequência nos primeiros seis meses após o transplante de órgãos sólidos.(6) Quando a ILTB for detectada, como ocorreu no nosso estudo, a prevenção com isoniazida é recomendada.(29)
Os transplantes com doador falecido com tempo de isquemia aumentado e retransplante são considerados como situações de alto risco imunológico. Nessas condições, indica-se a indução com fármacos mais potentes, como basiliximabe, timoglobulina ou outros anticorpos policlonais, no intuito de prevenir rejeição aguda e reduzir os efeitos da função retardada do enxerto tanto a curto quanto a longo prazo. Essa terapia aumenta o risco de desenvolvimento da tuberculose após o transplante e pode determinar uma resposta negativa a PT(4,6) comprometendo o diagnóstico de ILTB. (4,7) Porém, em nosso trabalho, essa associação com doador falecido e retransplante não foi evidenciada. Os transplantes renais premptivos e os transplantes duplos de órgãos mostraram uma tendência a maior positividade em relação a PT. Entretanto, deve-se considerar que esses representam uma pequena amostra, o que pode subestimar a análise.
Houve associações de história de contato com caso de tuberculose, presença da cicatriz para BCG e FER preservada com resultados positivos para a PT.
A chance de se apresentar PT positiva é 7,16 vezes maior quando o paciente relata história de contato com caso de tuberculose. A história de contato com tuberculose e sua associação com PT positiva é descrita de longa data e por vários autores, tendo, portanto, uma relação direta com o diagnóstico da ILTB.(3,9,13) No presente estudo, o contato prévio da doença mostrou uma associação significante com o resultado positivo da PT.
A presença de cicatriz da BCG aumenta em 3,07 vezes a chance de o paciente apresentar PT positiva. Já uma PT falso-positiva pode ocorrer pela vacina BCG recente.(30) Porém, estudos demonstram que não há interferência do resultado da PT se essa for realizada muitos anos após a vacinação,(13,31) uma vez que a resposta ao teste é praticamente nula e sem efeito 8-10 anos após a vacinação.(15,32) No presente estudo observou-se uma relação significante de vacinação BCG com positividade da PT. Todos os pacientes no nosso estudo que tinham cicatriz vacinal haviam sido vacinados há mais de 15 anos (média de idade de 46 anos). A vacinação prévia(13,15,32) é mais comumente considerada um fator de confusão do que de associação causal.
No nosso estudo utilizamos a isoniazida por seis meses para a prevenção da tuberculose; alguns estudos recomendam que seja realizada uma avaliação cuidadosa para decidir sobre utilização de outros fármacos na prevenção da doença.(2,6,8,33)
No presente estudo, a FER preservada (eGFR ≥ 60 mL/min/1,73 m2) foi a única variável dependente associada com resultado positivo para a PT. Os efeitos imunológicos resultantes da uremia, como alterações da fagocitose, labilidade bacteriana e transformação dos linfócitos, podem acarretar um resultado negativo da PT.(2,34) Assim, na FER reduzida, foram observados resultados negativos da PT, como descrito em outro trabalho.(28)
A prevalência da ILTB nos transplantados renais no nosso estudo (18,5%) foi menor que a encontrada por Sester et al.,(35) que obtiveram PT positiva em 52,14%, porém semelhante ao estudo de Atasever et al. (13,6%).(6) Isso se deve provavelmente ao fato de que o estado de MG registrou baixos coeficientes de incidência de tuberculose nos últimos anos.(36)
O incremento da PT2 em relação a PT1 (resposta significativa) demonstra que é aconselhável realizá-la sempre quando uma primeira reação for negativa, haja vista que a maioria não respondeu a PT1 (81%). Esse resultado já foi descrito em outros trabalhos quando a PT2 foi realizada,(5,13) o que favoreceu a detecção da ILTB nos pacientes em uso de imunossupressão. (8) Apesar de o Programa Nacional de Controle da Tuberculose indicar a PT em pacientes transplantados, observa-se que, com o incremento significativo da PT2, novos estudos deverão ser realizados incluindo outras populações para avaliar a reativação imunológica e indicar o segundo teste na prática clínica, pois, caso esses sejam positivos, deve-se orientar a utilização de medicamentos de prevenção e assim evitar o desenvolvimento da tuberculose.
Nosso estudo tem como limitação a utilização da PT, a qual pode não refletir a realidade da ILTB devido a imunodeficiência dos linfócitos e variação dos esquemas imunossupressores prescritos. Alguns autores estudam a possibilidade de novos marcadores para o diagnóstico de ILTB e tuberculose para resolver essa limitação, mas ainda não há evidências do uso de novos testes em transplantes de órgãos sólidos.(37,38)
Em conclusão, os fatores de risco observados para resultados positivos da PT na avaliação para ILTB em transplantados renais são contato com casos de tuberculose e FER preservada. A prevalência da ILTB foi baixa em transplantados renais. Uma PT2 deve ser realizada nesses pacientes quando a PT1 for negativa. A PT deve ser realizada quando houver melhoria na função renal.
AGRADECIMENTOSAgradecemos à Faculdade de Medicina da Universidade Federal de Minas Gerais e ao seu Grupo de Pesquisa em Micobacterioses.
REFERÊNCIAS1. Associação Brasileira de Transplante de Órgãos. Dimensionamento dos transplantes no Brasil e em cada estado (2009-2016). Registro Brasileiro de Transplantes [serial on the Internet]. 2016 [cited 2017 Oct 1]. XXII(4):[Adobe Acrobat document, 102p.]. Available from http://www.abto.org.br/abtov03/Upload/file/RBT/2016/RBT2016-leitura.pdf
2. Mu-oz P, Rodríguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis. 2005;40(4):581-7. https://doi.org/10.1086/427692
3. Lundin AP, Adler AJ, Berlyne GM, Friedman EA. Tuberculosis in patients undergoing maintenance hemodialysis. Am J Med. 1979;67(4):597-602. https://doi.org/10.1016/0002-9343(79)90240-7
4. Subramanian A, Dorman S; AST Infectious Diseases Community of Practice. Mycobacterium tuberculosis in solid organ transplant recipients. Am J Transplant. 2009; 9 Suppl 4:S57-62. https://doi.org/10.1111/j.1600-6143.2009.02894.x
5. Ergun I, Ekmekci Y, Sengul S, Kutlay S, Dede F, Canbakan B, et al. Mycobacterium tuberculosis infection in renal transplant recipients. Transplant Proc. 2006;38(5):1344-5. https://doi.org/10.1016/j.transproceed.2006.03.029
6. Atasever A, Bacakoglu F, Toz H, Basoglu OK, Duman S, Basak K, et al. Tuberculosis in renal transplant recipients on various immunosuppressive regimens. Nephrol Dial Transplant. 2005;20(4):797-802. https://doi.org/10.1093/ndt/gfh691
7. Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: Impact and implications for management. Clin Infect Dis. 1998;27(5):1266-77. https://doi.org/10.1086/514993
8. Torre-Cisneros J, Doblas A, Aguado JM, San Juan R, Blanes M, Montejo M, et al. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis. 2009;48(12):1657-65. https://doi.org/10.1086/599035
9. Subramanian AK, Morris MI; AST Infectious Diseases Community of Practice. Mycobacterium tuberculosis infectious in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:68-76. https://doi.org/10.1111/ajt.12100
10. Aguado JM, Herrero JA, Gavaldá J, Torre-Cisneros J, Blanes M, Rufí G, et al. Clinical presentation and outcome of tuberculosis in kidney, liver, and heart transplant recipients in Spain. Spanish Transplantation Infection Study Group, GESITRA. Transplantation. 1997;63(9):1278-86. https://doi.org/10.1097/00007890-199705150-00015
11. Fonseca JC, Caiaffa WT, Abreu MN, Farah Kde P, Carvalho Wda S, Spindola de Miranda S. Prevalence of latent tuberculosis infection and risk of infection in patients with chronic kidney disease undergoing hemodialysis in a referral center in Brazil. J Bras Pneumol. 2013;39(2): 214-20. https://doi.org/10.1590/S1806-37132013000200013
12. Guida JP, Bignotto Rosane D, Urbini-Santos C, Alves-Filho G, Ribeiro Resende M, Mazzali M. Tuberculosis in renal transplant recipients: a Brazilian center registry. Transplant Proc. 2009;41(3):883-4. https://doi.org/10.1016/j.transproceed.2009.01.075
13. Conde MB, Melo FA, Marques AM, Cardoso NC, Pinheiro VG, Dalcin Pde T, et al. III Brazilian Thoracic Association Guidelines on tuberculosis. J Bras Pneumol. 2009;35(10):1018-48. https://doi.org/10.1590/S1806-37132009001000011
14. World Health Organization [homepage on the Internet]. Geneva: WHO; c2017 [updated 2015; cited 2017 Oct 1] Guidelines on the management of latent tuberculosis infection; [about 2 screens]. http://www.who.int/tb/publications/ltbi_document_page/en/
15. Ruffino-Netto A. Interpretation of the tuberculin test [Article in Portuguese]. Rev Saude Publica. 2006;40(3):546-7. https://doi.org/10.1590/S0034-89102006000300026
16. Mayfield D, McLeod G, Hall P. The CAGE questionnaire: Validation of a new alcoholism instrument. Am J Psychiatry. 1974;131(10):1121-3.
17. Reichert J, Araújo AJ, Gonçalves CM, Godoy I, Chatkin JM, Sales MP, et al. Smoking cessation guidelines--2008. J Bras Pneumol. 2008;34(10):845-80. https://doi.org/10.1590/S1806-37132008001000014
18. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a World Health Organization Consultation. Geneva: World Health Organization; 2000.
19. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5-20. https://doi.org/10.2337/diacare.26.2007.S5
20. Métodos e critérios para o diagnóstico de Diabetes mellitus. In: Sociedade Brasileira de Diabetes. Tratamento e acompanhamento do Diabetes Mellitus--Diretrizes da Sociedade Brasileira de Diabetes. Rio de Janeiro: a sociedade; 2007. p. 14-5.
21. Buron F, Hadj-Aissa A, Dubourg L, Morelon E, Steghens JP, Ducher M, et al. Estimating glomerular filtration rate in kidney transplant recipients: performance over time of four creatinine-based formulas. Transplantation. 2011;92(9):1005-11. https://doi.org/10.1097/TP.0b013e3182301602
22. Reis-Santos B, Gomes T, Horta BL, Maciel ELN. Prevalência de tuberculose em transplantados renais: revisão sistemática e meta-análise. J Bras Nefrol. 2013;35(3):206-213. https://doi.org/10.5935/0101-2800.20130033
23. Van Zyl Smit RN, Pai M, Yew WW, Leung CC, Zumla A, Bateman ED, et al. Global lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. Eur Respir J. 2010;35(1):27-33. https://doi.org/10.1183/09031936.00072909
24. Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. The risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med. 2007;167(4):335-42. https://doi.org/10.1001/archinte.167.4.335
25. Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146(5):340-54. https://doi.org/10.7326/0003-4819-146-5-200703060-00006
26. Naqvi R, Akhtar S, Noor H, Saeed T, Bhatti S, Sheikh R, et al. Efficacy of isoniazid prophylaxis in renal allograft recipients. Transplant Proc. 2006;38(7):2057-8. https://doi.org/10.1016/j.transproceed.2006.06.010
27. Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80(4) 634-9. https://doi.org/10.4269/ajtmh.2009.80.634
28. Torres J, Aguado JM, San Juan R, Andrés A, Sierra P, López-Medrano F, et al. Hepatitis C virus, an important risk factor for tuberculosis in immunocompromised: experience with kidney transplantation. Transpl Int. 2008;21(9):873-8. https://doi.org/10.1111/j.1432-2277.2008.00694.x
29. Naqvi R, Naqvi A, Akhtar S, Ahmed E, Noor H, Saeed T, et al. Use of isoniazid chemoprophylaxis in renal transplant recipients. Nephrol Dial Transplant. 2010;25(2):634-7. https://doi.org/10.1093/ndt/gfp489
30. Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10(11):1192-204.
31. Brasil. Ministério da Saúde. Informe eletrônico da tuberculose. Boletim Eletrônico Epidemiológico. 2009;9(2):1-4.
32. Menzies D, Gardiner G, Farhat M, Greenaway C, Pai M. Thinking in three dimensions: a web-based algorithm to aid the interpretation of tuberculin skin test results. Int J Tuberc Lung Dis. 2008;12(5):498-505.
33. Currie AC, Knight SR, Morris PJ. Tuberculosis in renal transplant recipients: the evidence for prophylaxis. Transplantation. 2010;90(7):695-704. https://doi.org/10.1097/TP.0b013e3181ecea8d
34. Wauters A, Peetermans WE, Van de Brande P, De Moor B, Evenopoel P, et al. The value of tuberculin skin testing in haemodialysis patients. Nephrol Dial Transplant. 2004;19(2):433-8. https://doi.org/10.1093/ndt/gfg569
35. Sester U, Junker H, Hodapp T, Schütz A, Thiele B, Meyerhans A, et al. Improved efficiency in detecting cellular immunity towards M. tuberculosis in patients receiving immunosuppressive drug therapy. Nephrol Dial Transplant. 2006;21(11):3258-68. https://doi.org/10.1093/ndt/gfl416
36. Brasil. Ministério da Saúde. O controle da tuberculose no Brasil: avanços, inovações e desafios. Boletim Epidemiológico. 2014;45(2):1-13.
37. Kruh-Garcia NA, Schorey JS, Dobos KM. Exosomes: New Tuberculosis Biomarkers - Prospects From the Bench to the Clinic. In: Pere-Joan Cardona, editor. Understanding Tuberculosis: global experiences and innovative approaches to the diagnosis. Rijeka (Croatia): In Tech; 2012. p. 395-410.
38. Wallis R, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into prac-tice. Lancet. 2010;375(9729):1920-37. https://doi.org/10.1016/S0140-6736(10)60359-5




