ABSTRACT
Objective: Previous studies have demonstrated that closed pleural biopsy (CPB) has a sensitivity of less than 60% for diagnosing malignancy. Therefore, controversy has recently emerged regarding the value of CPB as a diagnostic test. Our objective was to assess the accuracy of CPB in diagnosing malignancy in patients with pleural effusion. Methods: This was a prospective 8-year study of individuals who underwent CPB to establish the etiology of pleural effusion. Information on each patient was obtained from anatomopathological reports and medical records. When CPB findings showed malignancy or tuberculosis, the biopsy was considered diagnostic, and that was the definitive diagnosis. In cases in which biopsy histopathological findings were nonspecific, a definitive diagnosis was established on the basis of other diagnostic procedures, such as thoracoscopy, thoracotomy, fiberoptic bronchoscopy, biochemical and cellular measurements in pleural fluid, and/or microbiological tests. The accuracy of CPB was determined with 2 × 2 contingency tables. Results: A total of 1034 biopsies from patients with pleural effusion were studied. Of those, 171 (16.54%) were excluded from the accuracy analysis either because of inadequate samples or insufficient information. The results of the accuracy analysis were as follows: sensitivity, 77%; specificity, 98%; positive predictive value, 99%; negative predictive value, 66%; positive likelihood ratio, 38.5; negative likelihood ratio, 0.23; pre-test probability, 2.13; and post-test probability, 82. Conclusions: CPB is useful in clinical practice as a diagnostic test, because there is an important change from pre-test to post-test probability.
Keywords:
Biopsy; Pleural effusion, malignant/diagnosis; Pleural effusion, malignant/epidemiology.
RESUMO
Objetivo: Estudios previos demuestran que la biopsia pleural cerrada (BPC) para diagnóstico de malignidad tiene una sensibilidad menor al 60%, por lo que recientemente ha despertado controversia su valor como prueba diagnóstica. Nuestro objetivo fue evaluar la exactitud de la BPC para diagnóstico de malignidad en pacientes con derrame pleural. Métodos: Estudio prospectivo de 8 años en individuos que se sometieron a la realización de BPC para establecer la etiología del derrame. La información de cada paciente se tomó de los registros de anatomopatología y del expediente clínico. Cuando el resultado de la BPC demostró malignidad o tuberculosis, esto se tomó como biopsia diagnóstica y quedó éste como diagnóstico definitivo. En los casos en que el resultado del estudio histopatológico de la biopsia resultó inespecífico, el diagnóstico definitivo se estableció en base a otros procedimientos diagnósticos, como toracoscopia, toracotomía, fibrobroncoscopia, estudio bioquímico y celular del líquido pleural y/o pruebas microbiológicas. Mediante una tabla de contingencia de 2 × 2 se midieron los indicadores para una prueba diagnóstica. Resultados: Se estudiaron 1034 biopsias de pacientes con derrame pleural, de las cuales se excluyeron 171 (16.54%) por muestra inadecuada o información insuficiente. El desempeño para malignidad fue: sensibilidad, 77%; especificidad, 98%; valores predictivos positivo y negativo, 99% y 66%, respectivamente; índices de probabilidad positivo y negativo, 38.5 y 0.23, respectivamente; probabilidad antes y después de la prueba, 2.13 y 82, respectivamente. Conclusión: La BPC es útil como prueba diagnóstica en la práctica clínica, debido a que produce un cambio importante de la probabilidad antes de la prueba a la probabilidad después de la prueba.
Palavras-chave:
Biopsia; Derrame pleural maligno, diagnóstico; Derrame plural maligno, epidemiología.
INTRODUCCIÓNEvidencia previa ha demostrado que el desempeño de la biopsia pleural cerrada ciega (BPC) como método de diagnóstico para malignidad es bajo, debido a que la sensibilidad reportada es menor al 60%, por lo que en algunos países se considera obsoleta y su uso tiende a desaparecer.(1,2)
Actualmente, el neumólogo intervencionista tiene dos opciones para la obtención de muestras histológicas de tejido pleural: la BPC o la toracoscopia médica. La primera es una técnica antigua(3,4) y la segunda, actualmente se considera el estándar de oro(2); sin embargo, ambos procedimientos tienen ventajas y desventajas. La BPC descrita por Cope y Abrams(3,4) a mediados del siglo XX es un método alternativo de obtención de tejido pleural sin la necesidad de un procedimiento quirúrgico. Su uso por más de 5 décadas se atribuye a la facilidad de su ejecución, bajo costo, tolerabilidad en el paciente y el hecho de que, en un período corto de tiempo, permite la decisión del manejo del caso.(5) No obstante, es un procedimiento puramente de diagnóstico, que no ofrece una ganancia en términos de tratamiento o alivio sintomático del paciente como ocurre con la toracoscopia médica o la videoasistida.
En nuestra institución se diagnostican aproximadamente 400 casos nuevos de neoplasia maligna intratorácica cada año, de los cuales, aproximadamente el 40% tiene derrame pleural. Su abordaje inicial es mediante la realización de toracocentesis para el análisis bioquímico y citológico del líquido pleural. En los casos en los que la toracocentesis no es diagnóstica y se trata de un exudado linfocítico, el diagnóstico definitivo se establece mediante análisis histopatológico de muestras obtenidas por BPC guiada o no guiada por imagen, o bien, por toracoscopia videoasistida.
Debido a que en nuestro hospital seguimos realizando la BPC en los casos de derrame pleural de tipo exudado linfocítico, del que no se sabía la etiología, el objetivo de este estudio fue evaluar la exactitud de la BPC para establecer el diagnóstico de malignidad en pacientes con derrame pleural en estudio.
MÉTODOSSe realizó un estudio prospectivo en pacientes con derrame pleural que se sometieron a la realización de BPC para establecer la etiología del derrame, durante un período de 8 años en un hospital de referencia de enfermedades respiratorias de la Ciudad de México. El estudio fue aprobado por el Comité de Ética en Investigación del Instituto Nacional de Enfermedades Respiratorias.
Previo a la realización de la BPC, se hizo estudio del líquido pleural que incluyó mediciones bioquímicas como niveles de proteínas, deshidrogenasa láctica y colesterol, para clasificar el derrame como exudado o trasudado,(6) y otras mediciones como nivel de glucosa, adenosín desaminasa y pH, así como evaluación del predominio de la celularidad y estudio citológico.
La BPC la realizaron distintos médicos neumólogos con 5 a 15 años de experiencia y médicos residentes de neumología de segundo o tercer año de los servicios de urgencias y hospitalización. Cuando la BPC fue ejecutada por médicos residentes, siempre fueron dirigidos y supervisados por el neumólogo responsable del paciente. El procedimiento de diagnóstico se realizó con aguja de Cope(3) o de Abrams(4) en pacientes con derrame pleural submasivo o masivo, en una sala de procedimientos de hospitalización o en el servicio de urgencias. Con el paciente en posición sentada, con los brazos al frente descansando sobre una mesa a la altura de sus hombros, en el lado afectado se identificó el punto de entrada, que generalmente fue entre la línea axilar posterior y la del ángulo inferior de la escápula, por el quinto espacio intercostal. Con técnica aséptica y anestesia local con lidocaína al 2% de la piel, tejido celular subcutáneo, músculo intercostal y región subyacente de pleura parietal, se realizó toracocentesis para asegurarnos de estar en el espacio pleural; posteriormente se realizó una incisión de 3 a 5 mm con una hoja de bisturí sobre la piel y tejido subcutáneo para facilitar la introducción de la aguja de biopsia sobre el trayecto del borde superior de la costilla subyacente, para evitar el daño del paquete vascular y nervioso intercostal.
La aguja de Abrams dispone de tres elementos: uno externo que hace de cánula de 11 G (3 mm de diámetro externo), un estilete interno de 13 G que facilita la introducción transparietal y una aguja de 13 G con gancho para toma de biopsia.
Al momento de la extracción de la aguja-estilete se evidenció salida de líquido pleural, con lo que confirmamos que estábamos en la cavidad pleural. Con la cánula en el espacio pleural, se introdujo la aguja-trócar hasta el espacio pleural a través de la cánula, la muesca del trócar se colocó en posición opuesta al grip de dicha aguja. Para coger la muestra de pleura parietal se trabó dicha muesca en la pleura, y con un movimiento giratorio de la aguja-trócar, se cortó y se obtuvo la muestra requerida. Estas acciones se repitieron tantas veces como muestras se desearon obtener.
La aguja de Cope dispone de cuatro elementos: una cánula externa 11 G de 3 mm de diámetro, una de 13 G con gancho de biopsia, una biselada, y una como estilete interno. Después de colocar una jeringa en el trócar con gancho-biopsia, se insertó el estilete interno en la biselada y ambas se insertaron a través de la cánula externa en el espacio pleural. A través de la cánula, se introdujo el trócar con el gancho de biopsia, cuyo orificio exterior tiene adaptada una jeringa. Comprobamos que estamos en el espacio pleural al aspirar fluido a través de la jeringa. El gancho de biopsia se introdujo en el trócar, se apoyó en la pleura parietal y con un ligero jalón hacia afuera se obtuvo la biopsia.
Con cualquiera de las dos agujas se tomaron entre 4 y 8 muestras en los sentidos horarios de las 3, 6 y 9, nunca en el de las 12 para evitar daño al paquete antes mencionado. Las biopsias se colocaron en solución de formaldehído al 3.7% para el examen histopatológico, el cual fue realizado por diferentes patólogos del departamento de patología con más de 10 años de experiencia en el diagnóstico de enfermedades pulmonares, pleurales y mediastinales. Inicialmente se realizó análisis morfológico de las biopsias y posteriormente se evaluaron mediante inmunohistoquímica, con los paneles de anticuerpos apropiados para el diagnóstico de las diferentes enfermedades neoplásicas y no neoplásicas.
La identificación de los casos en que se practicó la BPC se obtuvo de los registros del servicio de anatomopatología del hospital. La información de cada paciente, como características generales, antecedentes, cuadro clínico, hallazgos radiológicos, y resultados del estudio citológico y citoquímico del líquido pleural, se tomó de los expedientes clínicos de cada paciente durante su hospitalización.
Cuando el resultado de la BPC demostró malignidad o tuberculosis, esto se tomó como biopsia diagnóstica, quedando éste como diagnóstico definitivo en el expediente clínico. En los casos de exudado linfocítico con resultado histopatológico de la BPC con alteraciones inflamatorias inespecíficas (inflamación crónica inespecífica o hiperplasia mesotelial reactiva), el diagnóstico definitivo se estableció en base a otros procedimientos diagnósticos, que para estos casos fueron los siguientes: procedimientos invasivos como toracoscopia, toracotomía, fibrobroncoscopia, nivel de adenosín desaminasa y/o pruebas microbiológicas tanto para bacterias piógenas como para Mycobacterium tuberculosis; o bien el criterio clínico y estudios de laboratorio específicos de acuerdo al caso.
En todos los casos, los resultados del estudio histopatológico de la BPC se compararon con el diagnóstico definitivo emitido por el médico tratante y seguimiento de 6 meses, mediante el expediente clínico.
Se consideró tuberculosis pleural si se identificó al menos una de las siguientes evidencias: identificación de granulomas en la biopsia pleural; tinción de Ziehl-Neelsen positiva en líquido pleural o material de biopsia; el cultivo de líquido pleural en el medio de Löwenstein-Jensen positivo; baciloscopia en expectoración positiva; o adenosin desaminasa > 45 UI en un exudado pleural con predominio de linfocitos > 80%.
Se consideró derrame pleural paraneumónico cuando se observó un exudado pleural con predominio de polimorfonucleares además de un cuadro clínico compatible con neumonía; el caso se trató con antibióticos y hubo mejoría del cuadro al egreso del paciente.
Análisis estadísticoPara el análisis de exactitud de la BPC, se utilizaron tablas de contingencia 2 × 2 en donde se compararon los resultados de la prueba diagnóstica con respecto al estatus de cualquier enfermedad maligna intratorácica y de forma separada para mesotelioma.
Se calcularon la sensibilidad, especificidad, valores predictivos positivos y negativos, índices de probabilidad positivos y negativos y probabilidades antes y después de la prueba.(7)
RESULTADOSDurante el periodo de estudio se realizaron 1034 BPCs en el mismo número de pacientes con derrame pleural. La edad promedio fue de 57 ± 17 años, 615 (59.48%) fueron del sexo masculino y 419 (40.52%), del sexo femenino. Se identificó malignidad en 466 (45.07%) de los casos, entre los cuales el adenocarcinoma pulmonar y el mesotelioma fueron las neoplasias más frecuentes, encontradas en el 252 (24.37%) y en el 105 (10.16%) de los casos respectivamente (Tabla 1).

De las 1034 biopsias, se excluyeron del análisis de exactitud de la prueba a 171 (16.54%), de las cuales 72 (6.96%) fue por muestra inadecuada ya que no se obtuvo tejido pleural y 99 (9.57%), por falta de información del caso, debido a que no fue posible su seguimiento hasta el diagnóstico definitivo.
Los diagnósticos finales de los 863 casos incluidos en el análisis de exactitud resaltan con mayor frecuencia el adenocarcinoma pulmonar, el mesotelioma y el carcinoma indiferenciado (Tabla 2).
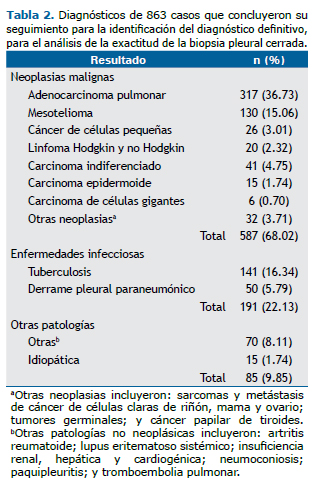
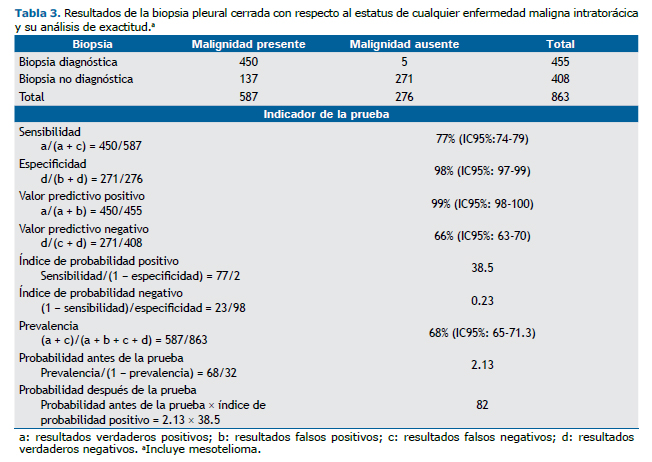
Los resultados de la BPC con respecto al estatus de cualquier enfermedad maligna intratorácica y para mesotelioma, así como los resultados de los indicadores del rendimiento de la prueba diagnóstica para cada caso, se describen en las tablas de contingencia 2 × 2 (Tablas 3 y 4).

En 38/863 (4.40%) de los casos se registraron complicaciones (3 casos presentaron dos complicaciones): 30 casos presentaron neumotórax, de los cuales 19 no requirieron la colocación de sonda endopleural y 11 si la requirieron; 6 pacientes presentaron hematoma en el sitio de la punción; 2, síncope vasovagal; y 3, enfisema subcutáneo alrededor de la zona de la punción.
DISCUSIÓNLos resultados de este estudio describen, conforme a la epidemiología clínica moderna, el rendimiento de la BPC en casos de derrame pleural de tipo exudado linfocítico y del que no se sabía la etiología. Esta prueba diagnóstica demostró ser útil ya que permitió identificar al 77% de los casos con derrame pleural debido a cualquier malignidad y al 81% debido a mesotelioma. El adenocarcinoma pulmonar metastásico a pleura y el mesotelioma fueron las neoplasias que se diagnosticaron más frecuentemente mediante este método. La especificidad de la prueba diagnóstica en estudio fue elevada, debido a que únicamente el 2% de los pacientes con otras causas de derrame pleural presentaron un resultado positivo para malignidad, y ninguno, para mesotelioma. Así mismo, en un paciente con un resultado de BPC positivo para enfermedad neoplásica, la probabilidad de que efectivamente tuviera neoplasia fue de 99%, y de 100% para mesotelioma. Sin embargo, en la práctica clínica es muy importante la confianza con la que es posible descartar la probabilidad de neoplasia torácica, dado un resultado normal o con alteraciones inflamatorias inespecíficas, en un escenario en donde la prevalencia de la enfermedad es elevada como es nuestro caso, de tal manera que un resultado negativo no excluye la probabilidad de neoplasia en el 34% de los casos. La utilidad de este procedimiento de diagnóstico radica en la posibilidad de excluir malignidad en los casos de derrame pleural de tipo exudado linfocítico ante un resultado de biopsia con inflamación crónica inespecífica o hiperplasia mesotelial reactiva. Nuestra serie y otras series publicadas refieren un número elevado de este tipo de resultados (20%-60%),(8-13) en los que subsecuentemente se confirma malignidad por otros métodos en algunos de ellos, siendo éstos los falsos negativos para malignidad. En el presente estudio 378 (36.56%) de las biopsias resultaron con alteraciones inflamatorias inespecíficas, y, en el análisis de exactitud, 137/587 (23.34%) correspondieron a resultados falsos negativos para malignidad.
La explicación de estos resultados falsos negativos se debe a que la diseminación de la neoplasia en la pleura en el caso de malignidad intratorácica es en parches, a que el derrame se debe a obstrucción de los linfáticos pleurales o mediastinales, o a que la afección sea solo de la pleura visceral. Entre las neoplasias que con mayor frecuencia dieron resultados falsos negativos en BPC fueron diversas variedades de sarcomas intratorácicos y las de origen mediastinal y metastásico fuera del tórax, como metástasis de cáncer de células claras de riñón, mama, ovario, tumores germinales y cáncer papilar de tiroides entre otros.
De acuerdo al resultado del índice de probabilidad positivo, un paciente con un exudado pleural linfocítico y un resultado de BPC positivo para malignidad tendrá 38.5 veces más probabilidad de padecer enfermedad maligna en el tórax, comparado con un paciente con las mismas características pero con un resultado de BPC negativo. Finalmente, existe una gran diferencia entre la probabilidad antes de la prueba y después de la prueba, 2.13 y 82 respectivamente, lo que sugiere un desplazamiento clínicamente importante. Una tendencia similar se observó en el caso de mesotelioma.
Los estudios previos realizados sobre el desempeño de la BPC para el diagnóstico de malignidad, ya sean sólo para BPC o comparados con los métodos guiados por imagen o por toracoscopia médica, demuestran que la BPC utilizando las agujas de Cope o de Abrams permite diagnosticar entre el 21% y 62% de los casos de neoplasia como causa del derrame pleural(1,8-12,14,15) (Tabla 5). Cuando la biopsia pleural es guiada por algún método de imagen, ya sea ultrasonido o tomografía computada, demuestra de forma consistente un mayor rendimiento, con valores de sensibilidad entre el 77% y 87.5%,(1,11,13,16,17) y cuando existe engrosamiento pleural mayor de 1 cm, la sensibilidad se incrementa a 95%, valor semejante a la sensibilidad cuando la biopsia se obtiene por toracoscopia.(16) En general, las especificidades y valores predictivos positivos son elevados tanto para la BPC como en aquella que se realiza guiada por imagen. En nuestro estudio, el resultado de la sensibilidad para enfermedad neoplásica (77%) fue mayor con respecto a los estudios previos y dentro del intervalo para el caso de la biopsia pleural guiada por algún método de imagen (Tabla 5), y, semejante a los estudios previos, la especificidad y valor predictivo positivo fueron elevados en el presente estudio. Esto probablemente se debe a que el lugar donde se realizó nuestro estudio es un hospital de referencia de enfermedades respiratorias y a que muchos de los casos llegan en etapas avanzadas de la enfermedad.
La toracoscopia médica junto con la toracoscopia videoasistida se consideran los métodos estándar de oro para la obtención de biopsias cuando hay derrame pleural debido a neoplasia.(5) Para el caso de la toracoscopia médica, la evidencia demuestra sensibilidades del 86.2 al 93.5%(12,14,16) (Tabla 5).
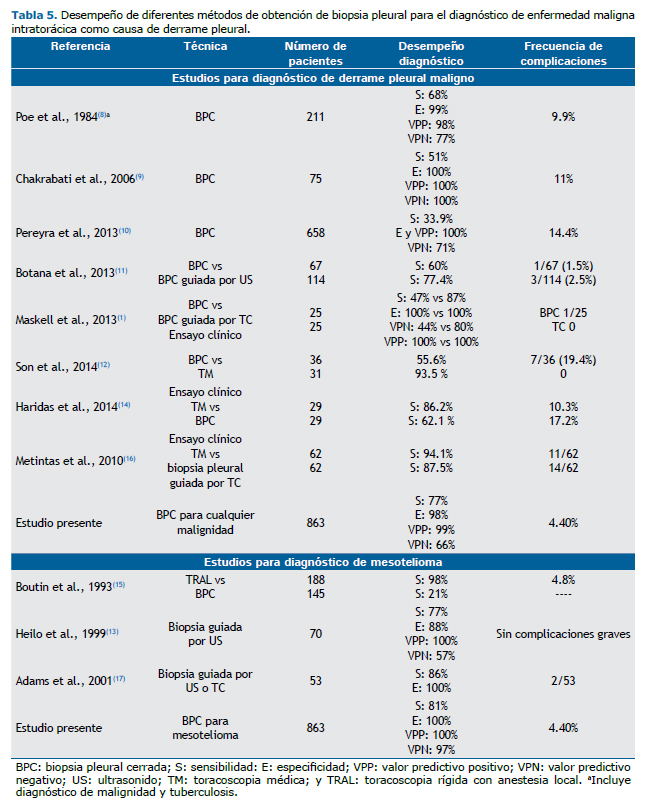
Los estudios que evalúan la exactitud de la BPC para el diagnóstico de mesotelioma son pocos. En un estudio se comparó su desempeño con la toracoscopia quirúrgica y mostró una sensibilidad de 21% vs 98 %.(15) Cuando la biopsia es guiada por algún método de imagen como ultrasonido o tomografía computada, la sensibilidad se eleva al 77% y 86%, respectivamente(13,17) (Tabla 5). Los resultados anteriores contrastan con los del presente estudio, debido a que los indicadores de desempeño de la BPC para el diagnóstico de mesotelioma fueron elevados, e inclusive semejantes cuando se comparan con los resultados de la biopsia guiada por algún método de imagen.(13,17) Este desempeño se explica en parte porque muchos de los casos presentaron engrosamiento pleural.
En nuestro estudio se confirma la seguridad del procedimiento y su accesibilidad inclusive para médicos neumólogos en formación como especialistas. La frecuencia de complicaciones fue de 4.40% (Tabla 5), semejante a lo que se ha informado en la literatura,(2) por otra parte se demuestra el desempeño de esta prueba en un escenario de trabajo rutinario, ya que tanto la obtención de las biopsias como su análisis histopatológico fue por diferentes operadores de forma independiente.
En nuestra institución, la BPC sigue presente en el algoritmo de diagnóstico de pacientes con derrame pleural de tipo exudado linfocítico para identificar la etiología, a pesar de que disponemos de la toracoscopia video-asistida cuyo desempeño con respecto a la BPC es mucho mejor, además de que facilita la realización de procedimientos terapéuticos de forma paralela a la obtención de biopsias como pleurodesis para prevenir la recurrencia del derrame; sin embargo, este procedimiento toma más tiempo, recursos y puede representar un riesgo en pacientes con riesgo anestésico elevado.
La toracoscopia médica aún no está disponible en nuestra institución, y las biopsias pleurales guiadas por imagen se realizan con menos frecuencia ya que se requiere del equipo, ya sea ultrasonido o tomografía computada, y de personal entrenado para ello. Actualmente en nuestra institución se trata de generalizar la realización de la BPC guiada por imagen, ya sea ultrasonido o tomografía computada, así como también implementar la utilización de la toracoscopia médica.
Entre las limitaciones del presente estudio, tenemos que no se registraron para el análisis los resultados de los estudios citológicos, cultivos ni hallazgos radiográficos como engrosamiento pleural, mismos que sólo se consideraron para la inclusión del caso al estudio y su diagnóstico definitivo. Otra limitante potencial es el número de casos excluidos del análisis, que sin duda pueden modificar los indicadores de la prueba; no obstante, consideramos que tenemos un buen tamaño de muestra que incluye a más del 80% de todos los casos registrados durante el período de estudio, por lo que estimamos que el cambio de los indicadores de la prueba sería modesto, sin modificar ostensiblemente la tendencia de los resultados.
En conclusión, en el sitio en donde se llevó a cabo el presente estudio, la BPC demostró ser válida, exacta y precisa para establecer el diagnóstico de malignidad intratorácica en pacientes con derrame pleural. Como prueba diagnóstica es útil en la práctica clínica, debido a que produce un cambio importante de la probabilidad antes de la prueba a la probabilidad después de la prueba.
REFERENCIAS1. Maskell NA, Gleeson FV. Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003;361(9366):1326-30. https://doi.org/10.1016/S0140-6736(03)13079-6
2. Dixon G, de Fonseka D, Maskell N. Pleural controversies: image guided biopsy vs. thoracoscopy for undiagnosed pleural effusions? J Thorac Dis. 2015;7(6):1041-51.
3. Cope C. New pleural biopsy needle; preliminary study. JAMA. 1958;167(9):1107-8. https://doi.org/10.1001/jama.1958.72990260005011a
4. Abrams LD. A pleural-biopsy punch. Lancet. 1958;1(7010):30-1. https://doi.org/10.1016/S0140-6736(58)92521-2
5. Koegelenberg CF, Diacon AH. Pleural controversy: closed needle pleural biopsy or thoracoscopy-which first? Respirology. 2011;16(5):738-46. https://doi.org/10.1111/j.1440-1843.2011.01973.x
6. Light RW. Clinical practice. Pleural effusion. N Engl J Med. 2002;346(25):1971-7. https://doi.org/10.1056/NEJMcp010731
7. Straus SE, Richardson WS, Glasziou P, Haynes RB. Diagnóstico y cribado. In: Straus SE, Richardson WS, Glasziou P, Haynes RB. 3rd edition, Medicina basada en la evidencia. Elsevier España; 2006; p. 67-100.
8. Poe R, Israel RH, Utell MJ, Hall WJ, Greenblatt DW, Kallay MC. Sensitivity, specificity, and predictive values of closed pleural biopsy. Ann Intern Med. 1984;144(2):325-8. https://doi.org/10.1001/archinte.1984.00350140139020
9. Chakrabarti B, Ryland I, Sheard J, Warburton CJ, Earis JE. The role of the Abrams percutaneous pleural biopsy in the investigation of exudative pleural effusions. Chest. 2006;129(6):1549-55. https://doi.org/10.1378/chest.129.6.1549
10. Pereyra MF, San-José E, Ferreiro L, Golpe A, Antúnez J, González-Barcala FJ, et al. Role of blind closed pleural biopsy in the management of pleural exudates. Can Respir J. 2013;20(5):362-6. https://doi.org/10.1155/2013/731352
11. Botana-Rial M, Leiro-Fernández V, Represas-Represas C, González-Piñeiro A, Tilve-Gómez A, Fernández-Villar A. Thoracic ultrasound-assisted selection for pleural biopsy with Abrams needle. Repir Care. 2013;58(11):1949-54. https://doi.org/10.4187/respcare.02378
12. Son HS, Lee SH, Darlong LM, Jung J, Sun K, Kim KT, et al. Is there a role for a needle thoracoscopic pleural biopsy under local anesthesia for pleural effusions? Korean J Thorac Cardiovasc Surg. 2014;;47(2):124-8. https://doi.org/10.5090/kjtcs.2014.47.2.124
13. Heilo A, Stenwig AE, Solheim OP. Malignant pleural mesothelioma: US-guided histologic core-needle biopsy. Radiology. 1999; 211(3):657-9. https://doi.org/10.1148/radiology.211.3.r99jn03657
14. Haridas N, Suraj KP, Rajagopal TP, James PT, Chetambath R. Medical Thoracoscopy vs Closed Pleural Biopsy in Pleural Effusions: A Randomized Controlled Study. J Clin Diagn Res. 2014;8(5):MC01-4.
15. Boutin C, Rey F. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 1: Diagnosis. Cancer. 1993;72(2):389-93. https://doi.org/10.1002/1097-0142(19930715)72:2<389::AID-CNCR2820720213>3.0.CO;2-V
16. Metintas M, Ak G, Dundar E, Yildirim H, Ozkan R, Kurt E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: a randomized, controlled trial. Chest. 2010;137(6):1362-8. https://doi.org/10.1378/chest.09-0884
17. Adams RF, Gleeson FV. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology. 2001;219(2):510-4. https://doi.org/10.1148/radiology.219.2.r01ma07510






