ABSTRACT
Objective: To use baseline audiogram parameters in order to ascertain whether drug-resistant tuberculosis (DR-TB) has effects on hearing, as well as to describe the configurations of the audiograms and to determine whether there are parameters that can be associated with those configurations. Methods: This was a prospective study involving patients diagnosed with DR-TB at a tuberculosis treatment center in the state of Ogun, in Nigeria. The patients included in the study were submitted to pure tone audiometry at baseline (within two weeks after treatment initiation). For comparative analyses, data regarding demographic and clinical characteristics were collected from the medical records of the patients. Results: The final sample comprised 132 patients. The mean age of the patients was 34.5 ± 12.6 years (range, 8-82 years), and the male:female ratio was 2:1. Of the 132 patients, 103 (78.0%) resided in neighboring states, 125 (94.7%) had previously experienced antituberculosis treatment failure, and 18 (13.6%) were retroviral-positive. Normal audiograms were found in 12 patients (9.1%), whereas sensorineural hearing loss was identified in 104 (78.8%), the two most common configurations being ascending, in 54 (40.9%), and sloping, in 26 (19.7%). Pure-tone averages at low frequencies (0.25-1.0 kHz) and high frequencies (2.0-8.0 kHz) were 33.0 dB and 40.0 dB, respectively. Regarding the degree of hearing loss in the better ear, 36 patients (27.3%) were classified as having normal hearing and 67 (50.8%) were classified as having mild hearing loss (26-40 dB), whereas 29 (21.9%) showed moderate or severe hearing loss. Among the variables studied (age, gender, retroviral status, previous treatment outcome, and weight at admission), only male gender was associated with audiometric configurations. Conclusions: In this sample of patients with DR-TB, most presented with bilateral, mild, suboptimal sensorineural hearing loss, and ascending/sloping audiometric configurations were associated with male gender.
Keywords:
Audiometry, pure-tone; Hearing loss, high-frequency; Drug-related side effects and adverse reactions; Tuberculosis, multidrug-resistant.
RESUMO
Objetivo: Utilizar parâmetros do audiograma basal para verificar se a tuberculose resistente (TB-R) tem efeitos na audição, descrever as configurações dos audiogramas e determinar se há parâmetros que possam ser associados a essas configurações. Métodos: Estudo prospectivo com pacientes diagnosticados com TB-R em um centro de tratamento de tuberculose no estado de Ogun, Nigéria. Os pacientes incluídos no estudo foram submetidos à audiometria de tons puros em até duas semanas após o início do tratamento (audiometria basal). Características demográficas e clínicas foram coletadas dos prontuários médicos dos pacientes para análises comparativas. Resultados: A amostra final envolveu 132 pacientes. A média de idade dos pacientes foi de 34,5 ± 12,6 anos (variação, 8-82 anos), e a razão homem:mulher foi de 2:1. A maioria dos pacientes (n = 103; 78,0%) residia nos estados vizinhos e tinha história de falha de tratamento antituberculose (n = 125; 94.7%); 18 (13.6%) apresentavam status retroviral positivo. Doze pacientes (9,1%) apresentaram audiogramas normais, e 104 (78,8%) apresentaram perda auditiva neurossensorial, sendo as configurações mais comuns do tipo ascendente, em 54 (40,9%), e descendente, em 26 (19,7%). As médias de tons puros em frequências baixas (0,25-1,0 kHz) e altas (2,0-8,0 kHz) foram de 33,0 dB e 40,0 dB, respectivamente. Quanto ao grau de perda auditiva no melhor ouvido, 36 pacientes (27,3%) apresentaram audição normal, e 67 (50,8%) apresentaram perda auditiva leve (26-40 dB), enquanto 29 (21,9%) mostraram perda auditiva moderada ou grave. Entre as variáveis estudadas (idade, gênero, status retroviral, desfecho de tratamento anterior e peso na admissão), somente o gênero masculino foi associado às configurações audiométricas. Conclusões: Nesta amostra de pacientes com TB-R, a maioria apresentou perda auditiva neurossensorial leve e subótima bilateralmente, com configurações audiométricas ascendentes/descendentes associadas ao gênero masculino.
Palavras-chave:
Audiometria de tons puros; Perda auditiva de alta frequência; Efeitos colaterais e reações adversas relacionadas a drogas; Tuberculose resistente a múltiplos medicamentos.
INTRODUÇÃOTuberculose resistente (TB-R) refere-se à infecção por um isolado de Mycobacterium tuberculosis resistente a um dos tuberculostáticos de primeira linha, a saber, isoniazida, pirazinamida, etambutol e estreptomicina. Com base em seu grau de resistência a esses medicamentos, as micobactérias podem ser classificadas em monorresistentes, multirresistentes (MRs) ou extensivamente resistentes. A prevalência de TB-R é variável, sendo que cepas resistentes a isoniazida e rifampicina (isto é, cepas TB-MR) foram identificadas em 0,6% a 2,9% dos casos novos de tuberculose diagnosticados na África Subsaariana.(1,2) Além disso, a TB-R é comum entre indivíduos infectados por retrovírus; a incidência de tuberculose resistente a rifampicina entre indivíduos infectados pelo HIV na Nigéria foi de 7.0% ao longo de um período de três anos (2009-2012).(3) O surgimento de cepas micobacterianas resistentes é um fator importante que alimenta a epidemia de tuberculose e sua morbidade e mortalidade associadas.(4)
Embora a TB-R possa ser uma infecção primária quando um paciente é infectado por uma cepa de M. tuberculosis já resistente, ela surge mais frequentemente por meio da seleção de cepas mutadas pela terapia inadequada. O mais poderoso preditor da presença de TB-R é a história de tratamento antituberculose.(5) Outros fatores, como a escassez de medicamentos, levam à inadequação do esquema inicial antituberculose e alimentam o surgimento da TB-R, especialmente em cenários com poucos recursos. Além do mais, o aumento dos custos do tratamento e as complicações associadas ao uso de medicamentos antituberculose contribuem consideravelmente para a complexidade do tratamento da TB-R.(5)
Os efeitos adversos do tratamento antituberculose incluem erupções cutâneas, distúrbios gastrintestinais e hepatotoxicidade, enquanto os aminoglicosídeos podem causar nefrotoxicidade e ototoxicidade.(6) O comprometimento auditivo neurossensorial foi observado como a forma mais comum de manifestação de ototoxicidade.(7) Isso é detectado pela monitoração dos parâmetros dos limiares auditivos em pacientes em uso de tuberculostáticos como parte do protocolo de manejo da TB-R. O comprometimento auditivo resultante da ototoxicidade é detectado pela comparação das medidas dos limiares auditivos durante o tratamento com a avaliação auditiva inicial obtida do paciente na admissão, antes do começo da terapia (audiometria basal). No entanto, a tuberculose pode afetar os ouvidos de outras formas como parte de suas manifestações extrapulmonares, causando infecções, como otite média tuberculosa, paralisia dos nervos faciais e diversos tipos e graus de perda auditiva (PA). (8) Os efeitos que as micobactérias têm na audição, diferentemente dos efeitos dos medicamentos, podem ser avaliados subjetivamente a partir da audiometria basal obtida antes do início do tratamento.
O objetivo do presente estudo foi utilizar parâmetros do audiograma basal para verificar se a TB-R tem efeitos na audição, descrever as configurações dos audiogramas e determinar se há parâmetros clínicos que possam ser associados a essas configurações. Acreditamos que isso irá auxiliar na caracterização inicial dos limiares auditivos e irá esclarecer os efeitos audiológicos da tuberculose.
MÉTODOSTrata-se de um estudo prospectivo envolvendo pacientes acompanhados no Centro de Tuberculose Resistente do Estado de Ogun no Sacred Heart Hospital (Special), localizado em Lantoro, Abeokuta, Nigéria, entre novembro de 2014 e outubro de 2015. O centro recebe pacientes com TB-R de seu estado de origem (Ogun) e de estados vizinhos (Lagos, Oyo, Ondo, Ekiti, Kwara, Kogi, Edo e Delta).
Os pacientes que foram diagnosticados com TB-R - por meio do GeneXpert MTB/RIF® (Cepheid, Sunnyvale, CA, EUA) e cultura de escarro positiva para M. tuberculosis - foram admitidos e tratados no centro. Os casos foram manejados por uma equipe de especialistas, que incluía um pneumologista, um otorrinolaringologista, clínicos (treinados no manejo da tuberculose), um psiquiatra, enfermeiros e um assistente social. O pneumologista realizou as avaliações iniciais dos pacientes e confirmou o diagnóstico de TB-R auxiliado pelos investigadores. Outros especialistas também desempenharam diferentes papéis no manejo dos casos. O protocolo do estudo foi aprovado pelo Comitê de Ética em Pesquisa do Sacred Heart Hospital, e todos os pacientes que participaram do estudo assinaram um termo de consentimento livre e esclarecido.
Como parte do protocolo de manejo da TB-R, o otorrinolaringologista avaliou a audiometria inicial (basal) dos pacientes na admissão ou em até duas semanas após a admissão e do início do tratamento para avaliar os limiares auditivos. O raciocínio por trás dessas avaliações e o significado das mesmas foram explicados aos pacientes, e apenas os que consentiram tiveram seus dados incluídos no estudo. Os pacientes foram questionados sobre doenças do ouvido - descargas do ouvido atuais, anteriores ou recorrentes - sintomas - PA, ruído nos ouvidos (zumbido), ecos e história de vertigem - e autoestimativa da audição - boa ou ruim - para a detecção de comprometimentos auditivos e dificuldade de audição. Os dois ouvidos foram então examinados com o uso de um otoscópio (HEINE Optotechnik, Herrsching, Alemanha) para a detecção de patologias, como membrana timpânica perfurada, descarga do ouvido, impactação de cera e presença de corpo estranho. Os pacientes com lesões do ouvido, como impactação de cera, foram tratados antes da realização da avaliação auditiva.
Os critérios de inclusão foram ter ouvidos anatomicamente normais ou apresentar anormalidades estruturais resultantes da infecção tuberculosa, bem como ter realizado audiometria em até duas semanas após o início do tratamento (audiometria basal). Os dados dos pacientes com sequela de perfuração de membrana timpânica suspeita de estar relacionada com otite média supurativa e não resultante de infecção tuberculosa foram excluídos do estudo.
Cada paciente foi posicionado sentado em uma sala silenciosa com nível de ruído ambiente de 27 dB (nível de pressão sonora), foi instruído sobre como realizar o teste e foi informado sobre o tipo de respostas esperado. Um audiômetro diagnóstico calibrado (Amplivox 240; Amplivox Ltd., Eynsham, Reino Unido), uma faixa de cabeça e um estimulador ósseo foram utilizados para produzir sons de tons puros, variando de 0,25-8,00 kHz e de 0,25-4,0 kHz, para a determinação dos limiares de condução aérea e de condução óssea, respectivamente. Os limiares auditivos foram medidos como intensidade sonora em decibéis com base nas respostas consistentes do paciente em cada frequência testada. As respostas foram representadas em forma de gráfico para a produção do audiograma para cada ouvido. Os tipos de audiogramas e a lateralidade também foram registrados. Os tipos de audiogramas foram classificados em normal, PA condutiva, PA neurossensorial ou PA mista. A PA neurossensorial foi subclassificada em descendente, plana ou ascendente, com base na forma dos traçados movendo-se das frequências mais baixas para as mais altas nos audiogramas. A lateralidade foi baseada no lado do audiograma anormal, e um audiograma discordante representou diferentes tipos de anormalidades nos dois ouvidos. Também foi observado o grau de PA no melhor ouvido em cada paciente. Adotamos a classificação de PA da Organização Mundial da Saúde, utilizando a média de tons puros, da seguinte forma: 0-25 dB, audição normal; 26-40 dB, PA leve; 41-60 dB, PA moderada; 61-80 dB, PA grave; e ≥ 81 dB, PA profunda.
Os parâmetros demográficos, como local habitual de domicílio, e os parâmetros clínicos, incluindo história de tratamento da tuberculose (cura ou falha) e status retroviral, foram obtidos dos prontuários médicos dos pacientes. Esses dados e os dados audiométricos foram inseridos em uma planilha e analisados com o programa IBM SPSS Statistics, versão 20.0 (IBM Corporation, Armonk, NY, EUA). As características descritivas dos pacientes foram resumidas em tabelas e apresentadas em números absolutos e proporções (variáveis categóricas) ou em médias e desvios padrão (variáveis contínuas). As variáveis (idade, gênero, status retroviral, falha de tratamento anterior e peso corporal) foram comparadas com as configurações audiométricas dos pacientes. O teste do qui-quadrado foi utilizado para detectar diferenças significativas entre as variáveis (p < 0,05).
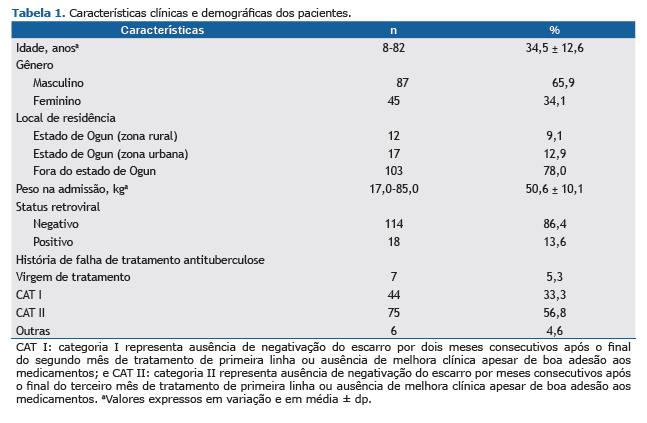
RESULTADOSDurante um período de um ano, 142 pacientes foram acompanhados. No entanto, 10 pacientes foram excluídos do estudo (5 não conseguiram realizar a audiometria basal por estarem demasiadamente fracos para suportar o estresse da avaliação audiométrica; 2 apresentaram respostas inconsistentes e não confiáveis durante a audiometria; e 3 foram submetidos à audiometria após duas semanas de tratamento). Portanto, a amostra foi composta por 132 pacientes. A média de idade dos pacientes foi de 34,5 ± 12,6 anos (variação, 8-82 anos). A razão homem:mulher foi de 2:1. Dos 132 pacientes, 103 (78,0%) não residiam no estado de Ogun e 18 (13,6%) apresentavam status retroviral positivo. O status auditivo autorrelatado foi o seguinte: 111 pacientes (84,1%) descreveram sua audição como boa; 13 (9,8%) ficaram indecisos, e 8 (6,1%) a descreveram como ruim. Falha de tratamento anterior de tuberculose (com diferentes combinações de drogas, incluindo rifampicina, isoniazida, pirazinamida e etambutol) foi relatada por 125 pacientes (94,7%). Após dois meses de tratamento de primeira linha, 44 pacientes (33,3%) não negativaram o escarro, enquanto após três meses de tratamento de primeira linha, 75 pacientes (56,8%) não negativaram o escarro e nem apresentaram melhora clínica apesar da boa adesão ao tratamento, conforme mostrado na Tabela 1.
A Tabela 2 apresenta as características audiométricas dos pacientes, mostrando que < 10% dos pacientes apresentaram audiogramas normais. Identificou-se PA neurossensorial em 104 pacientes (78,8%), entre os quais as configurações mais comuns foram do tipo ascendente (em 40,9%) e descendente (em 19,7%). As médias de tons puros (média aritmética dos limiares de condução aérea) em frequências baixas (0,25-1,0 kHz) e altas (2,0-8,0 kHz) foram de 33,0 dB e 40,0 dB, respectivamente.

A análise do grau de PA nos pacientes revelou que 27,3% apresentavam audição normal no melhor ouvido, metade (50, 8%) apresentava PA moderada (26-40 dB) e os demais apresentavam graus mais elevados de PA.
Na Tabela 3, foram explorados os fatores que poderiam estar associados às configurações audiométricas ascendente e descendente. Entre todos os fatores estudados, apenas o gênero masculino foi associado a ambas as configurações audiométricas, enquanto idade, status retroviral positivo, falha de tratamento anterior e peso corporal < 50,0 kg na admissão não foram associados às configurações audiométricas.
 DISCUSSÃO
DISCUSSÃOO presente estudo revelou que apenas 12 (9,1%) dos nossos pacientes com TB-R apresentavam audição normal em ambos os ouvidos, 36 (27,3%) apresentavam audição normal em um ouvido, e a maioria apresentava comprometimento auditivo em ambos os ouvidos. O principal tipo de comprometimento auditivo foi a PA neurossensorial - especialmente apresentando configurações audiométricas ascendentes ou descendentes, de magnitude leve - que foi associada ao gênero masculino. Assim, as prevalências de limiares auditivos aumentados, isto é, audição reduzida em nível basal em um ouvido e em ambos os ouvidos, foram, respectivamente, de 90,9% e 72,7%. Essas prevalências certamente são muito altas, levantando a suspeita de um efeito direto das micobactérias nos ouvidos de pacientes com TB-R. No entanto, isso deve ser interpretado com certa cautela, uma vez que esses resultados podem ser um reflexo da tendência geral de audição nessa comunidade, especialmente porque não houve controles para comparação. Além do mais, os níveis de poluição sonora nessa comunidade são altos,(10,11) e isso pode ter relação com os limiares auditivos médios nessa população.
A grande maioria (94,7%) dos nossos pacientes tinha história de falha de tratamento medicamentoso. Os esquemas de tratamento utilizados anteriormente, que variaram em conteúdo e tempo de uso, incluíram os tuberculostáticos padrão de primeira linha utilizados na Nigéria (rifampicina, isoniazida, pirazinamida e etambutol). Esses medicamentos foram utilizados em um esquema de seis meses, compreendendo uma fase intensiva de dois meses e uma fase de continuação de quatro meses. A possibilidade de que problemas auditivos anteriores poderiam ser agravados pelo uso de aminoglicosídeos é uma questão a ser explorada. Pesquisadores haviam relatado uma incidência de ototoxicidade de até 1.7% entre pacientes com tuberculose em uso de terapia combinada padrão com tuberculostáticos.(12) Portanto, os limiares auditivos aumentados encontrados no presente estudo podem estar parcialmente relacionados com os efeitos ototóxicos de um tratamento anterior com rifampicina ou isoniazida.
A prevalência de ototoxicidade entre pacientes com TB-R em tratamento na Europa e na Ásia foi de 28,0%(13) e 70,1%,(14) respectivamente. Vale observar que esses números citados não são valores audiométricos basais, e a ototoxicidade se baseia em critérios rigorosos, incluindo alterações audiométricas permanentes nos limiares auditivos, em frequências específicas. Pode ser suficiente afirmar que o nível de audição da maioria dos pacientes com TB-R em nossa coorte era subótimo. No entanto, uma preocupação mais séria em relação a essas altas prevalências de audição subótima são os efeitos e repercussões em longo prazo que elas poderiam ter na audição de pacientes que ainda vão estar em tratamento com aminoglicosídeos durante meses. Há uma tendência de agravamento da PA, com consequentes aumentos da proporção de pacientes com comprometimento auditivo. É conveniente considerar outras medicações além de aminoglicosídeos no tratamento da tuberculose, e pesquisas a esse respeito estão em andamento.
A maioria dos audiologistas define audiograma "basal" como aquele obtido em até duas semanas após o início do tratamento.(15) Isso é baseado na suposição de que alterações significativas nos limiares não teriam ocorrido em até duas semanas, mesmo se os pacientes tivessem iniciado a terapia. Além disso, a realização desse teste imediatamente após a admissão pode não ser possível por motivos de logística, e é melhor não atrasar o início da terapia em nosso cenário local. Apesar da exclusão dos dados dos pacientes com doenças do ouvido médio evidentes, como descarga do ouvido recorrente e membranas timpânicas perfuradas em decorrência de infecções supurativas no ouvido, 4,5% dos pacientes apresentaram evidências audiométricas de PA condutiva. É possível que isso se deva a deficiência subclínica do mecanismo de condução do ouvido médio. O tipo mais comum de padrão audiométrico encontrado em nossa amostra foi a PA neurossensorial, o que denotou o comprometimento das células ciliadas ou dos nervos cocleares. Há relatos de que a tuberculose afeta os nervos periféricos, causando neuropatias, especialmente durante os estágios tardios da infecção, embora a patogênese desse aspecto da doença permaneça controverso.(16) No entanto, diversos relatos sobre o comprometimento diferencial das células ciliadas cocleares se relacionam com patologias causadas por ototoxicidade induzida por medicamentos, bem como com PA relacionada à idade e PA induzida por ruído.
Ao se considerar audiogramas, três configurações de PA neurossensorial são descritas, a saber, configurações ascendente, plana e descendente. Em nosso estudo, a configuração mais comum foi a configuração ascendente, com PA em baixas frequências (PABF), também chamada de audiograma em "U" invertido. A PABF denota comprometimento de células ciliadas na volta apical da cóclea. Esse tipo de PA não é facilmente identificado por meio de sintomas em um cenário clínico, pois os sintomas são sutis, e, portanto, os audiologistas frequentemente o descrevem como assintomático. Os sons de baixa frequência são mais intensos e supostamente carregam menos informação do que os sons de alta frequência.(17) Uma pessoa com grau moderado de PABF pode não exibir nenhum sinal externo de PA, como não ouvir sons da fala ou ter padrões anormais de produção da fala. Assim, esses pacientes mantém uma inteligibilidade relativamente intacta. Além do mais, as informações de baixa frequência podem ser carregadas por fibras de alta frequência através da codificação temporal, limitando as pistas sugestivas de PABF a sintomas sutis, como dificuldades de audição em contexto grupal ou em local barulhento. Causas e fatores associados significativos para PABF incluem uma mutação do gene 1 da síndrome de Wolfram (uma doença autossômica recessiva denominada síndrome de Wolfram 1),(18) displasia de Mondini,(19) PA súbita,(20) doença de Ménière e infecções virais. (21) Pode ser precipitado supor que as micobactérias afetam as células ciliadas da mesma forma que as infecções virais, e, portanto, esse fenômeno necessita de maior esclarecimento.
A configuração audiométrica descendente, que representa a PA em altas frequências (PAAF), tem sido associada ao comprometimento auditivo induzido por ruído e à PA relacionada à idade (presbiacusia). Embora alguns dos pacientes de nossa amostra fossem idosos (≥ 60 anos de idade), nem todos os pacientes dessa faixa etária apresentavam PAAF. No entanto, as alterações auditivas relacionadas à idade tendem a começar a se manifestar por volta da quinta década de vida em nigerianos.(22) O avançar da idade e o alto nível de ruído em nossa comunidade podem ter causado PAAF em alguns dos pacientes de nossa amostra. A maior parte dos pacientes apresentou o mesmo padrão em ambos os ouvidos, sugerindo patologias semelhantes. Embora a tuberculose afete principalmente o parênquima pulmonar, seus efeitos em outros órgãos do corpo geralmente são sistêmicos. Os efeitos sistêmicos poderiam ser devidos à citopatia imunomediada ou à citopatia relacionada à vasculite,(16) e não à invasão direta de micobactérias nos ouvidos.
A maioria dos audiologistas está familiarizada com a classificação do grau de PA segundo a Organização Mundial da Saúde.(9) No presente estudo, a maioria dos pacientes apresentava grau leve de PA, que pode não ser perceptível no início. A exposição continua aos mesmos estímulos leva à progressão e piora da condição clínica dos pacientes, enquanto a administração de aminoglicosídeos injetáveis levará ao agravamento dos sintomas. Começando como uma alteração inicial temporária dos limiares, a ototoxicidade acaba levando a uma alteração permanente dos limiares, que é irreversível. Sugere-se, assim, que pacientes com qualquer forma de PA não devem tomar os aminoglicosídeos altamente ototóxicos desde o início, ou, alternativamente, esses pacientes devem passar por monitoração audiométrica com maior frequência para que se detectem alterações dos limiares rapidamente e se façam os adequados ajustes antes que haja alteração permanente dos limiares. No entanto, a praticabilidade dessas sugestões em ambientes com poucos recursos é duvidosa. Mesmo assim, o presente estudo reforça a necessidade de medições audiométricas iniciais dos limiares auditivos. Defendemos medições audiométricas seriadas para todos os pacientes em terapia para qualquer forma de infecção tuberculosa.
Enquanto o processo judicial contra o prestador de assistência médica pode não ser particularmente comum no momento, a possibilidade não pode ser descartada. Existem alguns pacientes com PA que necessitarão de amplificação sonora, aparelhos de auxílio auditivo ou reabilitação auditiva. Esses pacientes devem iniciar tratamento com um medicamento como a capreomicina, que parece apresentar menor toxicidade(13) do que outros aminoglicosídeos, como a canamicina. No entanto, esses pacientes precisam ser educados sobre seus problemas auditivos, a preexistência de PA antes do início da terapia deve ser enfatizada, e as ações necessárias precisarão ser tomadas. A assistência prontamente disponível que prestamos a esses pacientes incluiu aparelhos auditivos gratuitos.
A descoberta de fatores associados a uma doença pode auxiliar o clínico no manejo da doença. Entre os fatores que foram explorados sobre as duas configurações audiométricas mais comuns no presente estudo, apenas o sexo masculino se mostrou associado tanto à configuração audiométrica ascendente quanto à descendente. Embora isso possa sinalizar alguma influência hormonal, é possível que essa seja um supersimplificação da situação. Alguns estudos observaram limiares auditivos comparativamente aumentados nos homens (em comparação com as mulheres), especialmente naqueles entre 20 e 69 anos de idade.(23-25) Park et al. relataram que os limiares auditivos nas frequências de 3 kHz, 4 kHz e 6 kHz apresentaram diferença estatisticamente significativa entre os dois gêneros para pessoas com mais de 30 anos de idade, sendo que a frequência de 4 kHz foi responsável pela maior diferença significativa nessa amostra da população da Coreia do Sul.(26) Os fatores que foram atribuídos a isso incluem exposição a ruído ocupacional e de lazer, bem como o uso de analgésicos ou anti-inflamatórios não esteroidais, que ocorrem mais comumente em homens.(27)
Nenhum dos outros fatores estudados se mostrou associado às configurações ascendente ou descendente. Teoricamente, seria de se esperar que pacientes mais velhos, aqueles com história de falha de esquema medicamentoso, aqueles com menor peso corporal na admissão e aqueles coinfectados pelo HIV apresentassem piores limiares auditivos. Relatou-se, no entanto, que os riscos absolutos e os fatores de risco para eventos adversos (possivelmente incluindo PA) foram semelhantes entre pacientes infectados e não infectados pelo HIV tratados para TB-R em uma coorte de 57 pacientes na Namíbia.(28) Foi relatado que déficits auditivos em indivíduos infectados pelo HIV poderiam ser uma alteração auditiva central (e não periférica) que o audiograma de tons puros detecta. (29) De forma semelhante, pacientes infectados pelo HIV e virgens de tratamento antirretroviral não são mais susceptíveis a apresentar aumento de reações adversas graves aos medicamentos quando em uso de esquema antituberculose de segunda linha.(30) Isso reforça a necessidade de mais pesquisas sobre o assunto, especialmente quanto à ligação que resulta da TB-R e da infecção pelo HIV.
Os fatores associados ao comprometimento auditivo e às configurações audiométricas em pacientes com TB-R podem ser mais bem esclarecidos por meio de estudos controlados e aleatorizados. Esses estudos devem realizar análises comparativas entre indivíduos normais, pacientes com tuberculose e aqueles com TB-R. A falta desse tipo de análise continua sendo a principal limitação do presente estudo. Vale também observar que a avaliação do índice de massa corporal dos pacientes na admissão teria representado melhor o estado nutricional do que o peso corporal.(31) Além disso, a avaliação audiométrica basal deve idealmente ser realizada antes do começo da terapia. Mesmo considerando essas limitações, o presente estudo sugere que, na apresentação, a maioria dos pacientes com TB-R na Nigéria apresentava PA neurossensorial leve bilateralmente e configurações audiométricas ascendentes ou descendentes, sendo o gênero masculino o único fator associado a essas configurações.
REFERÊNCIAS1. Sanchez-Padilla E, Ardizzoni E, Sauvageot D, Ahoua L, Martin A, Varaine F, et al. Multidrug- and isoniazid-resistant tuberculosis in three high HIV burden African regions. Int J Tuberc Lung Dis. 2013;17(8):1036-42. https://doi.org/10.5588/ijtld.12.0842
2. Nigeria. Federal Ministry of Health. National DR-TB prevalence survey report. Abuja: Nigeria; 2011. p. 14-5.
3. Dinic L, Akande P, Idigbe EO, Ani A, Onwujekwe D, Agbaji O, et al. Genetic determinants of drug-resistant tuberculosis among HIV-infected patients in Nigeria. J Clin Microbiol. 2012;50(9):2905-9. https://doi.org/10.1128/JCM.00982-12
4. Singh N, Sidiq Z, Bhalla M, Myneedu VP, Sarin R. Multi-drug resistant tuberculosis among category I treatment failures--a retrospective study. Indian J Tuberc. 2014;61(2):148-51.
5. Marahatta SB Multi-drug resistant tuberculosis burden and risk factors: an update. Kathmandu Univ Med J (KUMJ). 2010;8(29):116-25. https://doi.org/10.3126/kumj.v8i1.3234
6. Zhang HQ, Xi XE, Wang YL, Han W, Zhang CX, Jiao JH. Side effects of tuberculosis treatment with fixed-dose combinations. J Biol Regul Homeost Agents. 2015;29(2):379-88.
7. Vasconcelos KA, Lima MA, Frota S, Ruffino Netto A, Kritski AL. Audiometric evaluation of patients treated for pulmonary tuberculosis. J Bras Pneumol. 2012;38(1):81-7. https://doi.org/10.1590/S1806-37132012000100012
8. Akkara SA, Singhania A, Akkara AG, Shah A, Adalja M, Chauhan N. A Study of Manifestations of Extrapulmonary Tuberculosis in the ENT Region. Indian J Otolaryngol Head Neck Surg. 2014;66(1):46-50. https://doi.org/10.1007/s12070-013-0661-7
9. World Health Organization [homepage on the Internet]. Geneva: World Health Organization [cited 2016 Jun 1]. Prevention of blindness and deafness. Grades of hearing impairment [About 2 screens]. Available from: http://www.who.int/pbd/deafness/hearing_impairment_grades/en/
10. Sogebi OA, Amoran OE, Iyaniwura CA, Oyewole EA. Awareness and attitudes to noise and its hazards in motor parks in a sub-urban Nigerian town. Niger Postgrad Med J. 2014;21(1):40-5.
11. Oyedepo OS, Saadu AA. A comparative study of noise pollution levels in some selected areas in Ilorin Metropolis, Nigeria. Environ Monit Assess. 2009;158(1-4):155-67. https://doi.org/10.1007/s10661-008-0570-5
12. Gülbay BE, Gürkan OU, Yildiz OA, Onen ZP, Erkekol FO, Baççioğlu A, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100(10):1834-42. https://doi.org/10.1016/j.rmed.2006.01.014
13. Sturdy A, Goodman A, José RJ, Loyse A, O'Donoghue M, Kon OM, et al. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother. 2011;66(8):1815-20. https://doi.org/10.1093/jac/dkr221
14. Javadi MR, Abtahi B, Gholami K, Safari Moghadam B, Tabarsi P, Salamzadeh J. The Incidence of Amikacin Ototoxicity in Multidrug-Resistant Tuberculosis Patients. Iran J Pharm Res. 2011;10(4):905-11.
15. Duggal P, Sarkar M. Audiologic monitoring of multi-drug resistant tuberculosis patients on aminoglycoside treatment with long term follow-up. BMC Ear, Nose Throat Disord. 2007; 7:5. https://doi.org/10.1186/1472-6815-7-5
16. Warpe BM, Poflee SV, Pande NP, Shrikhande AV. Tuberculous neuritis: a rare sequel of a common disease. Indian J Pathol Microbiol. 2014;57(1):69-71. https://doi.org/10.4103/0377-4929.130902
17. Salminen NH. Human cortical sensitivity to interaural level differences in low- and high-frequency sounds. J Acoust Soc Am. 2015;137(2):EL190-3. https://doi.org/10.1121/1.4907736
18. Gene identified for low-frequency hearing loss. Hear J. 2002;55(3):7-8. https://doi.org/10.1097/01.HJ.0000293475.07402.ee
19. Parving A. Inherited low-frequency hearing loss. A new mixed conductive/sensorineural entity? Scand Audiol. 1984;13(1):47-56. https://doi.org/10.3109/01050398409076257
20. Mattox D, Simmons E. Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1977;86(4 Pt 1):463-80. https://doi.org/10.1177/000348947708600406
21. Djupesland G, Flottorp G, Degré M, Stien R, Skrede S. Cochlear hearing loss and viral infection. Acta Otolaryngol. 1979;87(3-4):247-54. https://doi.org/10.3109/00016487909126416
22. Sogebi OA, Olusoga-Peters OO, Oluwapelumi O. Clinical and audiometric features of presbycusis in Nigerians. Afr Health Sci. 2013;13(4):886-92. https://doi.org/10.4314/ahs.v13i4.4
23. Dreisbach LE, Kramer SJ, Cobos S, Cowart K. Racial and gender effects on pure-tone thresholds and distortion-product otoacoustic emissions (DPOAEs) in normal hearing young adults. Int J Audiol. 2007;46(8):419-26. https://doi.org/10.1080/14992020701355074
24. Bahng J, Lee J. Hearing Thresholds for a Geriatric Population Composed of Korean Males and Females. J Audiol Otol. 2015;19(2):91-6. https://doi.org/10.7874/jao.2015.19.2.91
25. Baraldi Gdos S, de Almeida LC, Borges AC. Hearing loss in aging. Braz J Otorhinolaryngol. 2007;73(1) :58-64. https://doi.org/10.1016/S1808-8694(15)31123-X
26. Park YH, Shin SH, Byun SW, Kim JY. Age- and Gender-Related Mean Hearing Threshold in a Highly-Screened Population: The Korean National Health and Nutrition Examination Survey 2010-2012. PLoS One. 2016;11(3):e0150783. https://doi.org/10.1371/journal.pone.0150783
27. Dalton DS, Cruickshanks KJ, Wiley TL, Klein BE, Klein R, Tweed TS. Association of leisure-time noise exposure and hearing loss. Audiology. 2001; 40(1):1-9. https://doi.org/10.3109/00206090109073095
28. Sagwa E, Ruswa N, Musasa JP, Mantel-Teeuwisse AK. Adverse events during treatment of drug-resistant tuberculosis: a comparison between patients with or without human immunodeficiency virus co-infection. Drug Saf. 2013;36(11):1087-96. https://doi.org/10.1007/s40264-013-0091-1
29. Maro II, Moshi N, Clavier OH, MacKenzie TA, Kline-Schoder RJ, Wilbur JC, et al. Auditory impairments in HIV-infected individuals in Tanzania. Ear Hear. 2014;35(3):306-17. https://doi.org/10.1097/01.aud.0000439101.07257.ed
30. Van der Walt M, Lancaster J, Odendaal R, Davis JG, Shean K, Farley J. Serious treatment related adverse drug reactions amongst anti-retroviral naïve MDR-TB patients. PLoS One. 2013;8(4):e58817. https://doi.org/10.1371/journal.pone.0058817
31. Putri FA, Burhan E, Nawas A, Soepandi PZ, Sutoyo DK, Agustin H, et al. Body mass index predictive of sputum culture conversion among MDR-TB patients in Indonesia. Int J Tuberc Lung Dis. 2014;18(5):564-70. https://doi.org/10.5588/ijtld.13.0602




