ABSTRACT
Objective: To describe orofacial muscle function in patients with severe asthma. Methods: This was a descriptive study comparing patients with severe controlled asthma (SCA) and severe uncontrolled asthma (SUA). We selected 160 patients, who completed a sociodemographic questionnaire and the 6-item Asthma Control Questionnaire (ACQ-6), as well as undergoing evaluation of orofacial muscle function. Results: Of the 160 patients evaluated, 126 (78.8%) and 34 (21.2%) presented with SCA and SUA, respectively, as defined by the Global Initiative for Asthma criteria. Regardless of the level of asthma control, the most frequent changes found after evaluation of muscle function were difficulty in chewing, oronasal breathing pattern, below-average or poor dental arch condition, and difficulty in swallowing. When the sample was stratified by FEV1 (% of predicted), was significantly higher proportions of SUA group patients, compared with SCA group patients, showed habitual open-mouth chewing (24.8% vs. 7.7%; p < 0.02), difficulty in swallowing water (33.7% vs. 17.3%; p < 0.04), and voice problems (81.2% vs. 51.9%; p < 0.01). When the sample was stratified by ACQ-6 score, the proportion of patients showing difficulty in swallowing bread was significantly higher in the SUA group than in the SCA group (66.6% vs. 26.6%; p < 0.01). Conclusions: The prevalence of changes in the stomatognathic system appears to be high among adults with severe asthma, regardless of the level of asthma control. We found that some such changes were significantly more common in patients with SUA than in those with SCA.
Keywords:
Speech/physiology; Stomatognathic system/physiopathology; Asthma/complications; Deglutition disorders; Mastication/physiology.
RESUMO
Objetivo: Descrever os achados da avaliação miofuncional orofacial em pacientes com asma grave. Métodos: Estudo descritivo comparando pacientes com asma grave controlada (AGC) e asma grave não controlada (AGNC). Foram selecionados 160 participantes, que responderam a um questionário sociodemográfico e o Asthma Control Questionnaire com seis questões (ACQ-6) e realizaram avaliação miofuncional orofacial. Resultados: Na amostra estudada, 126 (78,8%) e 34 (21,2%) pacientes, respectivamente, apresentavam AGC e AGNC segundo os critérios da Global Initiative for Asthma. Independentemente do nível de controle da asma grave, as alterações mais frequentes observadas na avaliação miofuncional foram problemas de mastigação, padrão de respiração oronasal, estado de conservação da arcada dentária médio ou ruim e problemas na deglutição. Quando a amostra foi estratificada pelo VEF1 (% do previsto), os resultados foram significativamente maiores no grupo AGNC que no grupo AGC quanto a mastigação habitual com boca aberta (24,8% vs. 7,7%; p < 0,02), deglutição de água com dificuldade (33,7% vs. 17,3%; p < 0,04) e problemas de voz (81,2% vs. 51,9%; p < 0,01). Quando estratificada pelo ACQ-6, os resultados do grupo AGNC foram significativamente maiores que no grupo AGC quanto à deglutição de pão com dificuldade (66,6% vs. 26,6%; p < 0,01). Conclusões: A prevalência de alterações do sistema estomatognático parece ser alta em adultos com asma grave independentemente do nível de controle da doença. No grupo AGNC, algumas dessas alterações foram significativamente mais frequentes que no grupo AGC.
Palavras-chave:
Fala/fisiologia; Sistema estomatognático/fisiopatologia; Asma/complicações; Transtornos de deglutição; Mastigação/fisiologia.
INTRODUÇÃOA atualização de 2015 do guia da Global Initiative for Asthma (GINA)(1) indica que 10-40% dos pacientes com as-ma (alérgica ou não alérgica) podem apresentar sua doença associada com a rinite. Porém, um estudo realizado num centro de referência em Salvador (BA) encontrou 100% de associação entre asma e rinite alérgica.(2) A rinite alérgica, por sua vez, pode provocar obstrução nasal, com consequente respiração oral durante o repouso, mesmo com os indivíduos com asma grave em períodos de estabilidade.(3) A respiração oral poderá alterar as funções do sistema estomatognático (respiração, sucção, mastigação, deglutição e fala), as quais influenciam os aspectos vitais e sociais.(4)
A literatura tem demonstrado que a respiração oral em crianças e adultos com asma grave pode provocar altera-ções de estruturas e funções do sistema estomatognático, representadas, por exemplo, por maxila atrésica e palato em ogiva; interposição da língua aos arcos dentários; mordida aberta e cruzada; lábios hipotônicos e ocluídos com tensão; e padrões inadequados de respiração, mastigação e deglutição.(5-8)
As estruturas móveis e estáticas do sistema estomatognático atuam em conjunto e sincronismo com o objetivo de executar as funções de respiração, sucção, mastigação, deglutição e fala. Pode-se hipotetizar que uma estrutura alterada na via aérea superior poderá alterar a função correspondente, como, por exemplo, a ausência de unidades dentárias interferirá na mastigação. Estando uma estrutura ou uma função alterada, as demais estruturas e funções poderão desempenhar seus papeis de forma adaptada àquela nova condição, por exemplo, hipotonia de língua levando a alteração na execução dos movimentos da deglutição.
A asma grave pode ser identificada através do difícil controle ou resposta ao tratamento, bem como pela presença de pelo menos um dos seguintes indicadores: baixo controle dos sintomas, indicado através do Asthma Control Questionnaire (ACQ) > 1,5 ou do Asthma Control Test < 20; exacerbações frequentes, com necessidade de duas ou mais doses de corticoide sistêmico (> 3 vezes ao dia) no último ano; exacerbações graves, com ao menos uma hospitalização ou necessidade de ventilação mecânica no último ano; limitação do fluxo aéreo após uso do broncodi-latador com VEF1 < 80% do previsto; além de sintomas frequentes de asma noturna e limitação nas atividades físi-cas.(9-11) Pacientes com asma grave tendem a apresentar elevada frequência de rinite alérgica, a qual tem como um dos sintomas clínicos a obstrução nasal e, em consequência, respiração predominantemente oral. Logo, nessa condi-ção, os órgãos fonoarticulatórios estarão posicionados de forma inadequada, podendo levar ao comprometimento das funções de sucção, mastigação, deglutição e fala. Portanto, o objetivo do presente estudo foi descrever os acha-dos da avaliação miofuncional orofacial em pacientes com asma grave.
MÉTODOSTrata-se de estudo do tipo corte transversal, no qual a amostra foi selecionada de forma consecutiva, a partir de um centro de referência em asma grave - Programa de Controle da Asma e Rinite Alérgica na Bahia (ProAR) - em Salvador (BA). Foram obedecidos os seguintes critérios de inclusão: diagnóstico de asma grave de acordo com os critérios do Global Initiative for Asthma,(12) idade de 18-85 anos e ambos os sexos. Foram adotados os seguintes critérios de exclusão: presença de neuropatias, síndromes genéticas, cardiopatias, doenças debilitantes, traumas de face, déficit cognitivo ou dificuldade em entender e realizar os movimentos solicitados; história de cirurgia de cabeça e pescoço; e gestantes.
Dos 160 convidados a participar da pesquisa, todos responderam a um questionário sociodemográfico e ao ACQ com seis questões (ACQ-6), com ponto de corte de 1,5 para controle.(13) Essa avaliação miofuncional constou de observação da face e função oral, seguindo um protocolo validado.(8). Os dados do VEF1 foram obtidos através de consulta ao prontuário, com prazo máximo de doze meses de realização.
Para o cálculo do tamanho amostral da frequência da disfunção miofuncional em pacientes com asma grave foi uti-lizado o programa PEPI-Sample (Sagebush Press, Salt Lake City, UT, EUA) e os seguintes parâmetros: nível de con-fiança de 95%; prevalência estimada para alteração miofuncional na população em geral: 30-40%; população de onde foi retirada a amostra: aproximadamente 2.000 pacientes com asma grave cadastrados no ProAR; e 10% como diferença aceitável da prevalência. Para responder ao objetivo, o tamanho amostral foi de 145 pacientes, conside-rando a possibilidade de 10% de perdas; logo, a amostra calculada foi de 160 pacientes.
Para a tabulação e a análise dos dados, foi utilizado o programa estatístico IBM SPSS Statistics, versão 20.0 (IBM Corporation, Armonk, NY, EUA). As variáveis quantitativas foram expressas através de média e desvio-padrão ou mediana e amplitude interquartil. As variáveis qualitativas foram expressas através de frequências simples e relati-vas. Para a comparação de proporções, foi utilizado teste do qui-quadrado. Para a comparação de duas médias, foi utilizado o teste t de Student para amostras independentes. Os valores de p < 0,05 foram considerados significantes.
O presente estudo foi aprovado pelo Comitê de Ética em Pesquisas da Universidade Federal da Bahia (Protocolo: 088/2010; resolução aditiva n. 41/2013). Os pacientes, ao concordarem em participar do estudo, assinaram o termo de consentimento livre e esclarecido.
RESULTADOSPara a avaliação miofuncional orofacial em pacientes com asma grave foram convidados 160 pacientes adultos (idade ≥ 18 anos). Observando os critérios de classificação da asma pelo GINA,(12) 126 pacientes (79%) apresenta-ram asma controlada e 34 (21%), asma não controlada. A Tabela 1 apresenta os aspectos sociodemográficos dos pacientes com asma grave cadastrados no ProAR, com as informações de gênero, cor da pele, escolaridade, renda familiar, idade, IMC, dados espirométricos e questionário ACQ-6.
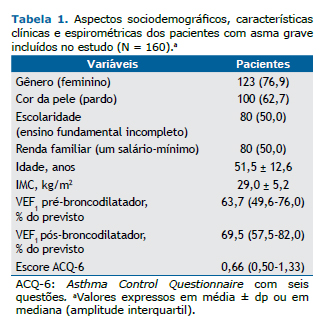
Dos 160 pacientes incluídos, no momento da avaliação miofuncional, 4 foram excluídos por não conseguirem exe-cutar os movimentos solicitados (3 com asma grave controlada e 1 com asma grave não controlada). A Figura 1 apresenta a descrição da avaliação do estado de conservação da arcada dentária, além da presença ou ausência de prótese dentária fixa ou móvel dos pacientes com asma grave cadastrados no ProAR, comparados de acordo com o controle da asma.
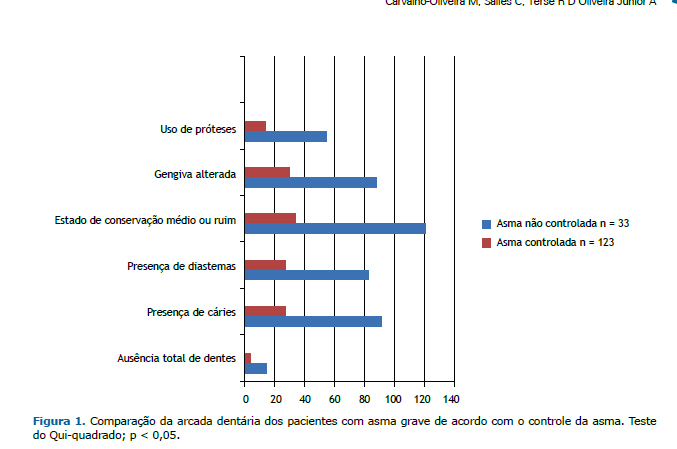
A Figura 2 apresenta os resultados da avaliação da função de mastigação de alimento sólido (pão de leite) dos pa-cientes com asma grave cadastrados no ProAR, comparados de acordo com o controle da asma.
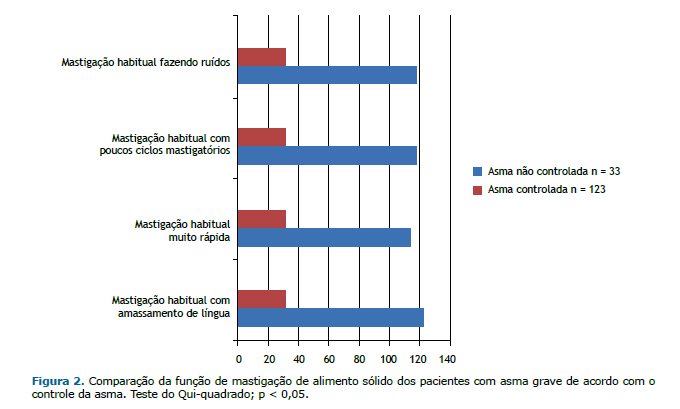
A Figura 3 apresenta os resultados da função de deglutição de sólido e de líquido dos pacientes com asma grave cadastrados no ProAR, comparados de acordo com o controle da asma.
Após a realização da avaliação miofuncional orofacial, coleta das respostas do questionário ACQ-6 e obtenção dos dados espirométricos, foram observadas alterações de respiração, voz, mobilidade de língua, função de mastigação e função de deglutição. Para um melhor entendimento dessas alterações fonoaudiológicas em pacientes asmáticos, as variáveis foram analisadas cruzando esses resultados com as duas medidas de controle da asma utilizadas. A Tabela 2 mostra o resultado da análise estatística, utilizando o teste do qui-quadrado, com a mobilidade do músculo língua, funções de mastigação e de deglutição e queixas vocais, comparando-as de acordo com a medida de VEF1 percentual, antes e depois do uso do broncodilatador.
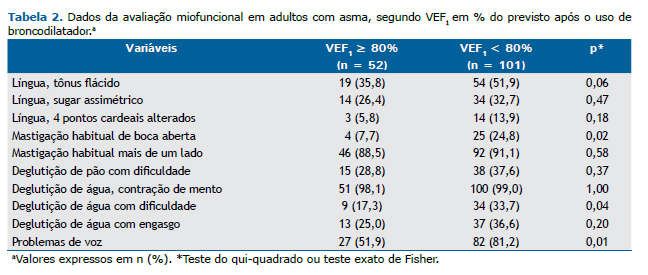
A Tabela 3 apresenta o resultado da análise estatística, utilizando o teste do qui-quadrado, com a mobilidade do músculo língua, funções de mastigação e de deglutição e queixas vocais, comparando-as de acordo com o controle da asma segundo o ACQ-6.
 DISCUSSÃO
DISCUSSÃOAtravés do presente estudo, foi possível observar que os pacientes com asma grave, tanto controlada como não controlada, apresentaram elevada frequência de alterações do sistema estomatognático. Foram usadas duas referên-cias como parâmetros de avaliação do controle da asma, uma objetiva e outra subjetiva. A espirometria é um exame objetivo e fornece os valores de VEF1 antes e depois do uso do broncodilatador. O ACQ é um questionário subjetivo para a avaliação do controle da asma que utiliza a memória e as percepções do paciente sobre o seu estado de saúde nos últimos sete dias. Os dois parâmetros estiveram associados com as variáveis estudadas.
Os resultados relacionados aos músculos e às funções do sistema estomatognático no presente estudo associaram-se com a gravidade da asma tanto com o VEF1 como com o ACQ-6. Campanha et al. também observaram que pacien-tes com asma não controlada apresentaram associações entre alterações do sistema estomatognático e VEF1.(14) Por outro lado, ao realizar-se tratamento fonoterápico para a adequação do padrão respiratório nasal em pacientes com asma, pode-se observar que a melhora clínica e funcional em relação ao padrão oronasal foi evidenciada pelo au-mento nos valores percentuais do PFE e do VEF1, apontando-se para a superioridade da respiração nasal.(15)
No presente estudo, as alterações vocais (Tabelas 2 e 3) foram frequentes em asmáticos e podem ser descritas como rouquidão, pigarro, voz arranhando, garganta seca, ardor ao falar, voz presa ou de difícil de produção. A litera-tura traz que o tratamento da asma pode afetar as vozes dos pacientes. O presente estudo corrobora o de Stanton et al., que concluíram que a qualidade vocal prejudicada é comum em doentes com asma, e que a escala Grade-Roughness-Breathiness-Asthenicity-Strain system (GRBAS, conhecida em português como RASATI) de avaliação vocal deve ser acrescentada à avaliação otorrinolaringológica e fonoaudiológica de pacientes asmáticos.(11)
Quanto à postura dos órgãos fonoarticulatórios, os achados encontrados no presente estudo foram os seguintes: língua habitualmente em posição anteriorizada; ponta da língua em posição baixa, em assoalho de boca; dorso da língua em posição rebaixada; maxila com padrão largo e alto; uso de prótese dentária; e úvula edemaciada e alon-gada. Corroborando esses resultados, Berlese et al. puderam observar diversas alterações orofaciais nos respirado-res orais, como lábios abertos e ressecados; lábio superior curto e hipofuncionante; lábio inferior com eversão e volumoso; língua hipotônica e rebaixada; maxila atrésica, com palato em ogiva; mordida aberta e cruzada; muscu-latura orofacial hipotônica; nariz achatado com narinas pequenas; protrusão dos dentes superiores, entre outras.(7)
Observou-se a ausência total de dentes em 18,3% dos entrevistados. Sobre a conservação da arcada dentária, foi possível observar cáries e diastemas nos dentes, independente da posição ocupada; estado de conservação ruim; gengiva alterada; e uso prótese dentária, móvel ou fixa. Num estudo realizado com crianças asmáticas em 2007, Shashikiran et al. encontraram uma associação entre o uso de medicação broncodilatadora, provocando efeitos locais da diminuição do pH salivar, e alteração dos níveis de secreção e composição salivares, justificando a elevada ocorrência de cáries e doenças periodontais, chamando a atenção para a necessidade de uma higiene mais efetiva como prevenção das cáries.(16) Outro estudo, relacionando asma com alterações ortodônticas, simetria facial e classi-ficação de Angle para oclusão dentária, observou a presença de crossbite, overbite e diastemas,(17) corroborando os achados encontrados no presente estudo.
As alterações na função de mastigação são amassamento de língua e mastigação rápida e pouca. Da Cunha et al. sugeriram que pacientes com asma tendem a mastigar em menor tempo. Dificuldades no processo respiratório e a incoordenação desse podem estar relacionados com a diminuição do tempo mastigatório, visto que essas pessoas apresentam dificuldades em manter o equilíbrio respiratório necessário durante o processo de alimentação.(18) A utilização da língua para ajudar na mastigação, promovendo o amassamento do alimento, corrobora com o resultado de Lemos et al., que mostram a mastigação como uma função aprendida, podendo sofrer modificações.(6) Os pacien-tes do presente estudo apresentaram muitos ruídos adventícios ao mastigar. Esse resultado pode estar relacionado com a elevada frequência de respiradores orais na população do estudo. Oliveira et al. definem a performance mas-tigatória como a mensuração da capacidade de fragmentar o alimento.(19) Acreditam que a obstrução nasal provoca ruídos e alterações na postura da língua, lábios e mandíbula. De tal modo, o respirador oral, assim como os asmáti-cos, não se alimenta bem, prejudicando seu desenvolvimento craniomaxilar e orofacial.
Os estudos das funções do sistema estomatognático atentam para o fato de que a idade na qual um indivíduo atin-ge o padrão maduro de deglutição é controverso, variando de 18 meses a 6 anos de idade. Lemos et al., em 2009, apontaram que existe a relação entre a respiração oral e a presença de alterações do padrão de deglutição.(6) Drozdz et al. relatam que o ato de deglutir depende de um processo complexo e dinâmico, utilizando estruturas comuns ao ato de respirar e, por isso, problemas respiratórios podem gerar dificuldades na deglutição.(20) Berlese et al. concordam sobre o fato de a respiração oral provocar alterações funcionais, como a deglutição adaptada, que pode ser caracterizada pela associação da ação labial, ação do músculo mentual e projeção lingual, que ocorre devido à diminuição de tônus e à postura rebaixada da língua.(7) Na tentativa de corrigir essas alterações, a muscu-latura perioral, incluindo os músculos orbiculares orais e o músculo mentual, atuam de forma mais ativa objetivando restabelecer a selagem labial necessária para a adequação da respiração.(7)
Salienta-se a importância do ineditismo do presente estudo, conduzido com adultos asmáticos graves. Pode-se considerar como limitações do presente estudo a ausência de um grupo controle; a amostra de conveniência coleta-da de forma consecutiva; a probabilidade de que as respostas subjetivas do ACQ-6 tenham influenciado negativa-mente para a correta percepção do controle da asma; e a ausência de um médico otorrinolaringologista para diag-nosticar e quantificar a presença de rinite alérgica. Entretanto, essa perda de informação está em acordo com dados da literatura.
Através do presente estudo, conclui-se que os pacientes com asma grave não controlada apresentaram maior fre-quência quanto às alterações do sistema estomatognático (músculos e estruturas), quando comparados com os paci-entes com asma controlada; que os pacientes com asma grave apresentaram elevada frequência de respiração oronasal, alterações em arcada dentária e alterações de voz; e que pacientes com asma grave apresentaram altera-ções do sistema estomatognático (funções de respiração, mastigação e deglutição), sendo que naqueles que tinham asma não controlada, essa frequência foi maior.
AGRADECIMENTOSAgradecemos à equipe do ProAR sua extrema solicitude e cooperação nos momentos de coleta de dados e aos pa-cientes com asma grave cadastrados no ProAR, tão disponíveis, sorridentes, interessados e colaborativos em todos os momentos da presente pesquisa.
REFERÊNCIAS1. Global Initiative for Asthma - GINA. [homepage on the Internet]. Bethesda: Global Initiative for Asthma. [cited 2015 Apr 1]. Pocket Guide for Health Professionals Updated 2015. [Adobe Acrobat document, 28p.]. Available from: http://ginasthma.org/wp-content/uploads/2016/01/GINA_Pocket_2015.pdf
2. Salles C, Cruz AA. Vias Aéreas Unidas. Rinite Alérgica: Conhecendo Melhor. 1st ed. São Paulo: Conexão Editorial; 2010, vol 1, p. 177-91.
3. Kairaitis K, Garlick SR, Wheatley JR, Amis TC. Route of breathing in patients with asthma. Chest. 1999;116(6):1646-52. http://dx.doi.org/10.1378/chest.116.6.1646
4. de Felicio CM, de Oliveira MM, da Silva MA. Effects of orofacial myofunctional therapy on temporomandibular disorders. Cranio. 2010;28(4):249-59. http://dx.doi.org/10.1179/crn.2010.033
5. Abreu RR, Rocha RL, Lamounier JA, Guerra AF. Prevalence of mouth breathing among children. J Pediatr (Rio J). 2008;84(5):467-70. http://dx.doi.org/10.1590/S0021-75572008000600015
6. Lemos CM, Wilhelmsen NS, Mion Ode G, Mello Júnior JF. Functional alterations of the stomatognathic system in patients with allergic rhinitis: case-control study. Braz J Otorhinolaryngol. 2009;75(2):268-74. http://dx.doi.org/10.1016/S1808-8694(15)30789-8
7. Berlese DB, Fontana PF, Botton L, Weimnann AR, Haeffner LS. Myofunctional characteristics of obese mouth and nose breathers. Rev Soc Bras Fonoaudiol. 2012;17(2):171-6. http://dx.doi.org/10.1590/S1516-80342012000200012
8. Castro MS, Toro AA, Sakano E, Ribeiro JD. Evaluation of oral functions of the stomatognathic system according to the levels of asthma severity. J Soc Bras Fonoaudiol. 2012;24(2):119-24. http://dx.doi.org/10.1590/S2179-64912012000200005
9. Fonseca JA, Botelho C. Severe asthma: definition [Article in Portuguese]. Rev Bras Alerg Imunopatol. 2006;29(2):70-6.
10. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-73. Erratum in: Eur Respir J. 2014;43(4):1216. http://dx.doi.org/10.1183/09031936.00202013
11. Stanton AE, Sellars C, Mackenzie K, McConnachie A, Bucknall CE. Perceived vocal morbidity in a problem asthma clinic. J Laryngol Otol. 2009;123(1):96-102. http://dx.doi.org/10.1017/S002221510800323X
12. Global Initiative for Asthma. Global strategy for asthma management and prevention. Bethesda: National Institutes of Health; 2011.
13. Leite M, Ponte EV, Petroni J, D'Oliveira Júnior A, Pizzichini E, Cruz AA. Evaluation of the asthma control questionnaire validated for use in Brazil. J Bras Pneumol. 2008;34(10):756-63. http://dx.doi.org/10.1590/S1806-37132008001000002
14. Campanha SM, Freire LM, Fontes MJ. Impact of asthma, allergic rhinitis and mouth breathing in life quality of children and adolescents. Rev CEFAC. 2008;10(4):513-9. http://dx.doi.org/10.1590/S1516-18462008000400011
15. Campanha SM, Fontes MJ, Santos JL. Dyspnea in patients with asthma, allergic rhinitis and mouth breathing. Rev CEFAC. 2012;14(2):268-73.
16. Shashikiran ND, Reddy VV, Raju PK. Effect of antiasthmatic medication on dental disease: dental caries and periodontal disease. J Indian Soc Pedod Prev Dent. 2007;25(2):65-8. http://dx.doi.org/10.4103/0970-4388.33450
17. Faria VC, de Oliveira MA, Santos LA, Santoro IL, Fernandes AL. The effects of asthma on dental and facial deformities. J Asthma. 2006;43(4):307-9. http://dx.doi.org/10.1080/02770900600623305
18. da Cunha DA, da Silva HJ, Nascimento GK, da Silva EG, da Cunha RA, Régis RM, et al, Analysis of the masticatory process of asthmatic children: Clinical and electromyographic research. Int Arch Otorhinolaryngol. 2012;16(3):358-64.
19. Oliveira RL, Noronha WP, Bonjardim LR. Masticatory performance evaluation in patients with nasal and mouth breathing [Article in Portuguese]. Rev CEFAC. 2012;14(1):114-21. http://dx.doi.org/10.1590/S1516-18462011005000112
20. Drozdz DR, Costa CC, Jesus PR, Trindade MS, Weiss G, Neto AB, et al. Pharyngeal swallowing phase and chronic cough. Int Arch Otorhinolaryngol. 2012;16(4):502-8.







