ABSTRACT
Objective: Many patients with proportional reductions in FVC and FEV1 on spirometry show no reduction in TLC. The aim of this study was to evaluate the role that measuring lung volumes and airway resistance plays in the correct classification of patients with a possible restrictive pattern on spirometry. Methods: This was a prospective study involving adults with reduced FVC and FEV1, as well as an FEV1/FV(C) ratio within the predicted range. Restrictive lung disease (RLD) was characterized by TLC below the 5th percentile, as determined by plethysmography. Obstructive lung disease (OLD) was characterized by high specific airway resistance, significant changes in post-bronchodilator FEV1, or an FEF25-75% < 50% of predicted, together with a high RV/TLC ratio. Nonspecific lung disease (NLD) was characterized by TLC within the predicted range and no obstruction. Combined lung disease (CLD) was characterized by reduced TLC and findings indicative of airflow obstruction. Clinical diagnoses were based on clinical suspicion, a respiratory questionnaire, and the review of tests of interest. Results: We included 300 patients in the study, of whom 108 (36%) were diagnosed with RLD. In addition, 120 (40%) and 72 (24%) were diagnosed with OLD/CLD and NLD, respectively. Among the latter, 24 (33%) were clinically diagnosed with OLD. In this sample, 151 patients (50.3%) were obese, and obesity was associated with all patterns of lung disease. Conclusions: Measuring lung volumes and airway resistance is often necessary in order to provide an appropriate characterization of the pattern of lung disease in patients presenting with a spirometry pattern suggestive of restriction. Airflow obstruction is common in such cases.
Keywords:
Spirometry; Airway resistance; Lung volume measurements.
RESUMO
Objetivo: Muitos pacientes com redução proporcional de CVF e VEF1 na espirometria não têm CPT reduzida. O objetivo deste estudo foi avaliar o papel da medida dos volumes pulmonares e da resistência das vias aéreas para a classificação correta de pacientes com possível restrição à espirometria. Métodos: Estudo prospectivo de adultos com CVF e VEF1 reduzidos e relação VEF1/CV(F) na faixa prevista. Distúrbio ventilatório restritivo (DVR) foi definido por CPT < 5º percentil por pletismografia. Distúrbio ventilatório obstrutivo (DVO) foi caracterizado por resistência específica de vias aéreas elevada, resposta significativa do VEF1 pós-broncodilatador e/ou um FEF25-75% < 50% do previsto associado a uma relação VR/CPT elevada. Distúrbio ventilatório inespecífico (DVI) foi caracterizado por CPT na faixa prevista e ausência de obstrução. Distúrbio ventilatório combinado (DVC) foi caracterizado por CPT reduzida e achados indicativos de obstrução ao fluxo aéreo. Os diagnósticos clínicos foram baseados em suspeita clínica, um questionário respiratório e revisão de exames de interesse. Resultados: Foram incluídos 300 pacientes no estudo, dos quais 108 (36%) tiveram diagnóstico de DVR, enquanto 120 (40%) foram diagnosticados com DVO ou DVC e 72 (24%) com DVI. Destes últimos, 24 (33%) tinham diagnóstico clínico de DVO. Nesta amostra, 151 pacientes (50,3%) eram obesos, e isso se associou com todos os padrões de distúrbios funcionais. Conclusões: Medidas dos volumes pulmonares e da resistência das vias aéreas são frequentemente necessárias para a caracterização adequada do tipo de distúrbio funcional em casos com possível restrição à espirometria. A obstrução ao fluxo aéreo é comum nesses casos.
Palavras-chave:
Espirometria; Resistência das vias respiratórias, Medidas de volume pulmonar.
INTRODUÇÃOA força-tarefa da American Thoracic Society (ATS)/European Respiratory Society (ERS) sugeriu definições para os diversos distúrbios funcionais.(1) Distúrbio ventilatório restritivo (DVR) foi definido pela redução da CPT abaixo do 5º percentil do valor previsto com relação VEF1/CV normal. Distúrbio ventilatório obstrutivo (DVO) foi definido por uma relação VEF1/CV abaixo do 5º percentil do valor previsto. Distúrbio ventilatório misto ou combinado (DVC) foi carac-terizado por ambos, VEF1/CV e CPT abaixo do 5º percentil dos valores previstos.
A redução da CV com a relação VEF1/CV(F) preservada é utilizada para inferir a presença de DVR; entretanto, em torno de 40% desses casos, não há redução da CPT. (2,3) A força-tarefa da ATS/ERS sugeriu que a presença de CV(F) reduzida, relação VEF1/CV(F) acima do limite inferior do normal e CPT na faixa prevista caracterizariam doença obstrutiva.(1) Esta anormalidade funcional foi posteriormente denominada distúrbio ventilatório inespecífico (DVI).(4) Em uma amostra de 100 pacientes com essa combinação, em 68 casos havia evidência de doença de vias aéreas, enquanto o restante mostrava sinais de restrição.(4)
A redução proporcional da CVF e do VEF1 em doenças obstrutivas pode ser explicada pelo fechamento de vias aé-reas, com aprisionamento aéreo.(5) A obesidade, ao reduzir mais a CV(F) do que o VEF1,(6) pode resultar em uma relação VEF1/CV(F) preservada na presença de doença obstrutiva concomitante. A obesidade, isoladamente, bem como doenças que afetam a mecânica respiratória ou a força dos músculos da respiração, também pode resultar em DVI.(4) Embora a DPOC e a asma respondam pela maioria das doenças pulmonares obstrutivas, um amplo espectro de outras doenças, incluindo doenças bronquiolares e algumas doenças intersticiais, são associadas com obstrução ao fluxo aéreo, podendo resultar em uma redução proporcional da CVF e do VEF1.(7) Além disso, pacientes com doen-ças pulmonares diversas são ou foram fumantes, o que pode contribuir com um componente obstrutivo.
A espirometria é considerada o método primário para a detecção de limitação ao fluxo aéreo decorrente de doen-ças pulmonares obstrutivas. Contudo, a limitação ao fluxo aéreo é o resultado final de muitos fatores. Um desses fatores é a resistência das vias aéreas (Rva) elevada.(8) A medida da Rva poderia levar à detecção de obstrução ao fluxo aéreo em muitos pacientes com possível restrição à espirometria. É usual afirmar que a Rva é um parâmetro pouco sensível em doenças que envolvem as vias aéreas periféricas; porém, um estudo clássico mostrou uma corre-lação estreita da condutância das vias aéreas (Gva) com o diâmetro bronquiolar.(9) A Gva pode ser anormal isola-damente na bronquiolite.(10) Em 2012, valores de referência para a resistência específica foram derivados em uma grande amostra.(11)
O objetivo do presente estudo foi avaliar o papel da medida dos volumes pulmonares e da Rva para a classificação funcional final de pacientes com possível restrição à espirometria.
MÉTODOSA coleta dos dados foi realizada nos laboratórios de função pulmonar do Centro Diagnóstico Brasil (n = 217) e do Hospital do Servidor Público Estadual de São Paulo (n = 83), no período entre dezembro de 2011 e dezembro de 2013. Pneumologistas certificados em função pulmonar pela Sociedade Brasileira de Pneumologia e Tisiologia (SBPT) e a autora principal do estudo selecionaram de forma prospectiva as espirometrias com possível restrição. O diagnóstico clínico foi definido pelo pneumologista solicitante do exame, pela aplicação de um questionário respira-tório padronizado e adaptado (Anexo 1: http://www.jornaldepneumologia.com.br/detalhe_anexo.asp?id=46)(12) e pela revisão ou indicação de exames complementares adicionais, como radiografia e tomografia de tórax, ecocardi-ograma, etc., de acordo com a suspeita clínica. Os exames de função pulmonar foram realizados por técnicas certifi-cadas (SBPT).(13) Todos os pacientes assinaram o termo de consentimento livre e esclarecido.
Critérios de inclusãoOs critérios de inclusão foram os seguintes: 1) indivíduos adultos com idade e estatura dentro da faixa de referên-cia das equações utilizadas(14); 2) redução da CVF, definida por valores situados abaixo do limite inferior do previsto, definido pelo 5º percentil da população de referência(14); 3) relação VEF1/CVF e VEF1/CV igual ou acima do limite inferior do previsto, definido pelo 5º percentil da população de referência(14); 4) diagnóstico clínico final definido (O diagnóstico de asma foi baseado no diagnóstico clínico feito por um médico e no relato de dois ou mais episódios de sibilância aliviados com broncodilatador. O diagnóstico de DPOC se baseou igualmente no médico assistente, na presença de tosse crônica e/ou dispneia pelo escore da escala do Medical Research Council ≥ 2, associados a taba-gismo pregresso ou atual. Os pacientes com diagnóstico de obesidade foram encaminhados, na maioria dos casos, para avaliação pré-operatória de cirurgia bariátrica, ou devido à queixa de dispneia, sem preencherem critérios para outros diagnósticos, tais como asma); e 5) testes de função pulmonar com critérios de aceitabilidade e reprodutibili-dade adequados (SBPT/ATS/ERS).(13,15-17)
Foram excluídos os pacientes com testes inadequados e os casos sem diagnóstico definido ou com diagnóstico du-vidoso ao final da análise.
Os testes de função pulmonar foram realizados em um sistema Sensor Medics 6200 Bodybox e em um sistema Col-lins (Ferraris Respiratory, Louisville, CO, EUA). Os volumes pulmonares foram determinados através de pletismogra-fia de corpo inteiro. Os valores previstos para os volumes pulmonares foram os sugeridos por Crapo et al.(18) A CPT reduzida foi caracterizada por valores situados abaixo do 5º percentil. Tanto o VR como a relação VR/CPT elevados foram caracterizados por valores acima do 95º percentil dos valores de referência.(18). As espirometrias foram repeti-das após a aplicação de broncodilatador (400 µg de salbutamol spray). A resposta ao broncodilatador considerada significativa foi aquela proposta por Soares et al. (VEF1 ≥ 0,20 l e 7% do previsto).(19)
A Rva foi medida pelos valores médios por interpolação linear, como sugerido por Matthys et al., após a análise de pelo menos cinco alças de pressão/fluxo adequadas.(20) Foram aceitas apenas alças satisfatórias e reprodutíveis. Os valores previstos utilizados para o cálculo foram os sugeridos por Piatti et al.(11) Valores acima de 8,0 cmH2O/s em mulheres e acima de 8,6 cmH2O/s em homens foram considerados elevados (média ± 1,64 dp).
Medidas adequadas da DLCO pelo método da respiração única foram obtidas em 260 pacientes. Os valores de re-ferência foram baseados em Miller et al.(21)
Após a obtenção de todos os dados, os distúrbios ventilatórios foram classificados em quatro grupos:
Grupo DVR: definido por CPT abaixo do limite inferior do previsto, sem achados de obstrução associada.(1)
Grupo DVO: caracterizado por um ou mais dos seguintes: Rva específica, corrigida para o volume pulmonar (Rva × Vp) elevada; variação significativa pós-broncodilatador (ΔVEF1 > 0,20 l e 7% do previsto); e/ou FEF25-75% < 50% do previsto associado à relação VR/CPT elevada (ver seção Resultados).
Grupo DVC: caracterizado quando achados dos dois grupos acima se encontravam combinados, isto é, CPT reduzi-da e Rva × Vp elevada; FEF25-75% < 50% associado à relação VR/CPT elevada; ou resposta significativa ao broncodi-latador.
Grupo DVI: caracterizado por CPT na faixa prevista na ausência de dados funcionais indicadores de obstrução.
Os valores foram expressos como média ± desvio-padrão. As comparações entre os grupos foram feitas por meio do teste t de Student e por ANOVA, no caso de variáveis independentes contínuas, e através do teste do qui-quadrado para variáveis nominais. Correlações entre Rva × Vp e parâmetros funcionais foram realizadas pelo teste de Spearman. A Rva × Vp exibiu distribuição log-normal, e seus valores foram transformados para comparações. A análise de curvas ROC foi utilizada para correlacionar os parâmetros funcionais e a relação VR/CPT com a resistên-cia específica de vias aéreas. A análise estatística foi realizada com pacote estatístico IBM SPSS Statistics, versão 20 (IBM Corp., Armonk, NY, EUA). O nível de significância foi estabelecido em α = 0,05.
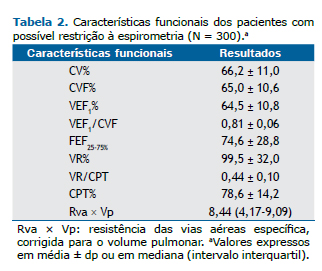
RESULTADOSForam incluídos 300 pacientes no estudo. As características gerais desses são mostradas na Tabela 1, enquanto os valores médios ± dp dos testes de função pulmonar, na Tabela 2.

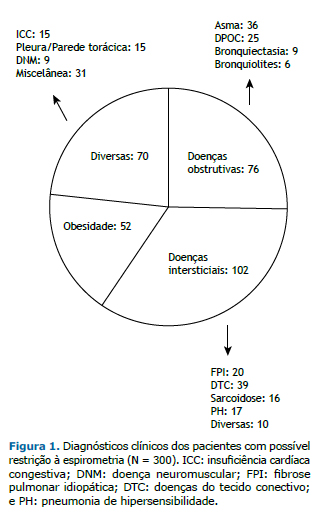
Os diagnósticos clínicos do total de pacientes foram separados em quatro grupos: doenças obstrutivas, doenças intersticiais, obesidade e diversas, conforme detalhado na Figura 1.
Dos 300 pacientes, 151 (50,3%) eram obesos, mas apenas 52 (17,3%) tiveram diagnóstico final de obesidade sem outras condições associadas. Dos 300 pacientes, 172 (57,3%) tinham CPT abaixo do limite inferior do previsto (DVR), e 128 (42,7%) tinham CPT na faixa prevista (n = 127) ou elevada (em apenas um caso).
O VR situou-se acima do limite superior em 46 (15,3%) e a relação VR/CPT em 126 (42,0%). Elevada Rva, corrigi-da para o volume pulmonar, foi observada em 97 (32,3%) dos casos. A correlação da Rva × Vp (e Gva/Vp) foi mais elevada com o FEF25-75% (rs = 0,55) em comparação à relação VEF1/CVF (rs = 0,50) e VEF1 em % do previsto (rs = 0,27; p < 0,01 para todos). A Rva × Vp também se correlacionou significativamente com a relação VR/CPT (rs = 0,46; p < 0,001). Por análise de curvas ROC, o FEF25-75% teve a maior área sob a curva (0,75; p < 0,001) para separar os paci-entes com Rva × Vp elevada daqueles com Rva × Vp não elevada, em comparação à relação VEF1/CVF e ao VEF1 em % do previsto. O FEF25-75% abaixo de 50% teve sensibilidade de 40% e especificidade de 89% para detectar resistên-cia específica elevada. Dentre os volumes pulmonares, a relação VR/CPT teve a maior área sob a curva para carac-terizar obstrução ao fluxo aéreo (0,75; p < 0,01). Como a relação VR/CPT elevada e o FEF25-75% < 50% podem ser observados de maneira isolada em DVR ou DVI, eles foram associados para caracterizar obstrução ao fluxo aéreo. Esses dois parâmetros estavam anormais em conjunto em 46 casos. Desses, tais parâmetros foram o único dado indicativo de distúrbio obstrutivo em 14 casos.
Respostas significativas pós-broncodilatador à espirometria foram observadas em 23 casos (7,7%). Os diagnósti-cos clínicos mais comuns nesse grupo foram doenças obstrutivas (n = 12) e obesidade (n = 5). Apenas um caso de insuficiência cardíaca congestiva (ICC) dentre 14 (7,0%) respondeu significativamente ao broncodilatador.
Por um ou mais dos critérios anteriormente apresentados, 120 pacientes (40,0%) tinham obstrução ao fluxo aéreo. Dos 120 pacientes com DVO, 64 (53,3%) tinham CPT abaixo do limite inferior do previsto, sendo considerados, portanto, portadores de DVC. Dos 128 casos com CPT situada na faixa prevista, 72 (56,2%) não tinham obstrução ao fluxo aéreo, sendo, portanto, classificados como portadores de DVI.
A classificação final dos tipos de distúrbios encontrados e os diagnósticos clínicos respectivos são mostrados na Figura 2. Os pacientes com diagnóstico de obesidade e com doenças variadas se distribuíram por todos os quatro grupos. Observa-se que 4 pacientes com diagnóstico de asma tinham DVR, enquanto 17 pacientes no grupo DVC tinham diagnóstico de doença obstrutiva, mais frequentemente asma (n = 10).
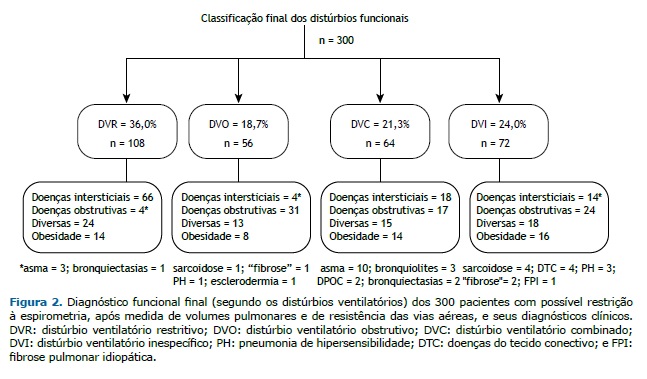
Nos 72 pacientes com diagnóstico funcional final de DVI, 24 (33,3%) tinham diagnóstico clínico de doença obstruti-va: asma, em 11; DPOC, em 8; bronquiectasias, em 3; e bronquiolite, em 3. Somando-se esses 24 pacientes aos 120 com diagnóstico funcional de DVO e DVC, 144 dos 300 casos (48,0%), portanto, teriam doença obstrutiva.
Diversas variáveis, incluindo idade, sexo e resultados funcionais, foram comparadas entre os quatro grandes gru-pos de diagnóstico clínico (Tabela 3). O grupo com diagnóstico final de obesidade era mais jovem e com índice de massa corpórea acima de 35 kg/m2 em 79% dos casos, refletindo que a maioria foi encaminhada para avaliação de cirurgia bariátrica. Funcionalmente, o grupo com diagnóstico de obesidade apresentava menor volume de reserva expiratório (VRE) em comparação aos demais grupos; valores percentuais de CV, CVF, VR e CPT maiores do que o grupo com doenças intersticiais; DLCO mais preservada; e Rva × Vp semelhante ao do grupo com doenças intersticiais.
 DISCUSSÃO
DISCUSSÃOO presente estudo confirma estudos anteriores(2-4) que mostraram que o padrão de redução da CVF e do VEF1 com a relação VEF1/CVF preservada à espirometria tem um valor limitado no diagnóstico funcional final. Além disso, o estudo mostra que, incorporando medidas de volumes pulmonares e de Rva, a caracterização funcional torna-se mais consistente.
É amplamente reconhecido que, para a caracterização de redução da CVF à espirometria, os critérios de esforço e o tempo expiratório adequados devem ser preenchidos. Uma expiração incompleta resulta frequentemente em pos-sível restrição à espirometria. No presente estudo, os testes foram cuidadosamente realizados e revistos.
A força-tarefa da ATS/ERS definiu DVR como uma redução da CPT abaixo do 5º percentil do valor previsto com relação VEF1/CVF não reduzida.(1) Os valores de referência utilizados para a CPT são, portanto, de grande importân-cia. No presente estudo, foram adotados os valores de Crapo et al.(18) Embora um estudo tenha derivado valores de referência para os volumes pulmonares em uma amostra da população no Brasil,(22) o número de indivíduos incluí-dos foi pequeno. Não podemos excluir a possibilidade de que pacientes com doenças obstrutivas que foram classifi-cados no presente estudo como portadores de DVR ou DVC seriam mais bem classificados com a disponibilidade de uma equação mais adequada para a determinação dos volumes pulmonares previstos. Entretanto, casos de asma com verdadeira restrição (CPT reduzida) não associados à obesidade são descritos na literatura, incluindo aqueles com variações na função pulmonar. (23) Esses casos são por vezes encontrados na prática clínica.
Doenças pulmonares restritivas podem se dever a doenças intersticiais, como fibrose pulmonar; a condições não respiratórias que secundariamente impedem a expansão pulmonar, como fraqueza muscular, doenças pleurais, obesidade ou cifoescoliose; e/ou às que afetam diretamente a função pulmonar, como a ICC. Diversos estudos têm encontrado uma prevalência de DVR na população, definida por espirometria, de 7-14%.(24-26) A prevalência é maior no sexo masculino, em fumantes pesados, em idosos, em pessoas com menor nível educacional, em diabéticos, naqueles com ICC e em indivíduos nos extremos de índice de massa corpórea. Grandes fumantes não raramente têm doenças intersticiais relacionadas ao tabaco, podendo resultar em padrão restritivo ou misto.(27)
A epidemia de obesidade não poupa países em desenvolvimento. A obesidade introduz fatores de confusão na in-terpretação da função pulmonar por diversos motivos. A obesidade afeta as medidas dos volumes pulmonares e os valores espirométricos, com especial ênfase na redução da capacidade residual funcional, por redução do VRE.(28) No presente estudo, o VRE foi significativamente menor no grupo com diagnóstico de obesidade. Reduções proporci-onais da CVF e do VEF1 com resultante preservação ou discreta elevação da relação VEF1/CVF têm sido observadas em indivíduos obesos. Contudo, as reduções da CVF e do VEF1, embora estatisticamente significantes, são tipicamen-te pequenas, e o VEF1 e a CVF, bem como a CPT, usualmente permanecem dentro da faixa de valores previstos.(6,28)
Em nosso estudo, metade da amostra era constituída por obesos. A presença de obesidade foi observada nos qua-tro tipos de distúrbios funcionais. A interação entre obesidade, função pulmonar, asma e DPOC tem sido objeto de diversos estudos e excelentes revisões. (6,29-31) A obesidade se associa com um risco aumentado para asma.(30,31).A presença de dispneia em obesos pode ser atribuída à obesidade ou à presença de asma, resultando tanto em diag-nósticos excessivos como em subdiagnósticos.(32) O teste de broncoprovocação induzida por metacolina é útil nesses casos.(4)
A medida dos volumes pulmonares poderia ser de auxílio na separação dos distúrbios restritivo e obstrutivo em pacientes com possível restrição à espirometria. (1) Um estudo mostrou que a concordância entre os diagnósticos clínicos e aqueles baseados nos testes de função pulmonar, com a adição das medidas de volumes pulmonares, é baixa para diferenciar DVR de DVO.(33)
No presente estudo, a inclusão de medidas da Rva e a combinação do FEF25-75% com relação VR/CPT elevada permi-tiu o diagnóstico de obstrução ao fluxo aéreo em diversos casos, com concordância significativa com os diagnósticos clínicos. Devido a considerações geométricas, onde a área total de secção transversal das vias aéreas diminui dra-maticamente da periferia para as regiões centrais do pulmão, medidas de resistência teoricamente seriam menos sensíveis a alterações periféricas. Contudo, a medida da Rva específica pode ser útil. Um estudo mostrou que a medida da Gva foi mais sensível que a espirometria para detectar obstrução ao fluxo aéreo em pacientes com a síndrome de bronquiolite obliterante.(10)
Na DPOC, na qual o local de obstrução situa-se perifericamente e em muitos casos com obstrução leve, a relação VEF1/CVF é reduzida e a Rva ou a Gva específica está na faixa prevista, mas casos na prática de laboratório de fun-ção pulmonar mostram que o contrário também ocorre. Um estudo realizado há quase 30 anos mostrou que a análise dos dados obtidos por pletismografia associado a dados clínicos detectou isoladamente 18% de casos com obstrução ao fluxo aéreo.(34) Um estudo clássico mostrou, em 26 pulmões submetidos à necropsia obtidos de vítimas de morte súbita, uma correlação hiperbólica quase perfeita entre o diâmetro bronquiolar médio e a Rva (r = 0,89), enquanto a correlação entre o diâmetro médio dos brônquios segmentares e a Rva não foi significativa. (9) No presente estudo, Rva × Vp elevada se associou significativamente com a redução do FEF25-75% e a elevação da relação VR/CPT, suge-rindo uma correlação com a obstrução de vias aéreas periféricas. Todos os casos tinham relação VEF1/CVF na faixa prevista.
Semelhante ao observado no grupo com obesidade como diagnóstico final, o grupo com diagnósticos diversos dis-tribuiu-se pelas quatro categorias diagnósticas funcionais. Nesse grupo de doenças, situavam-se pacientes com ICC, doenças de pleura e de parede (especialmente cifoescoliose), doenças neuromusculares e outras condições varia-das. Dos 102 pacientes com doenças pulmonares intersticiais, apenas 66 (65,0%) tinham confirmação de DVR isola-do pela medida da CPT. Os demais tinham DVO isolado (n = 4), DVC (n = 18) ou DVI (n = 14). Enfisema acompa-nhando fibrose pulmonar é uma condição relativamente comum, já que ambos se relacionam com o tabagismo.(35) Nas doenças do tecido conectivo, bronquiolites ou enfisema associado à doença intersticial e fraqueza muscular podem resultar em um componente obstrutivo ou inespecífico, respectivamente.(36,37) Na pneumonia de hipersensibi-lidade e na sarcoidose, o envolvimento de vias aéreas é frequente, podendo resultar em um componente obstruti-vo.(38,39)
No estudo de Hyatt et al.,(4) 68% dos pacientes com DVI tinham diagnóstico final de doença obstrutiva, corroboran-do a diretriz da ATS/ERS que a redução proporcional da CVF e do VEF1 com a CPT na faixa prevista indicaria doença obstrutiva. Entretanto, no grupo com doenças de vias aéreas, muitos pacientes tinham relação VEF1/CV lenta reduzi-da. No presente estudo, esses casos foram excluídos, e, ao final, apenas um terço dos portadores de DVI tinha diag-nóstico clínico de doença obstrutiva.
A seleção da amostra para a inclusão no presente estudo é uma limitação para a generalização dos resultados. Os pacientes incluídos não foram consecutivos, devido ao grande fluxo de casos atendidos na rotina. Eventuais diagnós-ticos funcionais discordantes do diagnóstico clínico podem ter ocorrido, visto que nem todos os exames para a inves-tigação de causas adicionais de restrição foram aplicados. Entretanto, o objetivo do estudo foi, a nosso ver, alcança-do.
Em conclusão, medidas dos volumes pulmonares e da Rva são de auxílio para a caracterização adequada do dis-túrbio funcional em diversos casos com possível restrição à espirometria. Doenças com obstrução ao fluxo aéreo podem resultar em padrão restritivo na espirometria.
REFERÊNCIAS1. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-68. http://dx.doi.org/10.1183/09031936.05.00035205
2. Aaron SD, Dales RE, Cardinal P. How accurate is spirometry at predicting restrictive pulmonary impairment? Chest. 1999;115(3):869-73. http://dx.doi.org/10.1378/chest.115.3.869
3. Venkateshiah SB, Ioachimescu OC, McCarthy K, Stoller JK. The utility of spirometry in diagnosing pulmonary restriction. Lung. 2008;186(1):19-25. http://dx.doi.org/10.1007/s00408-007-9052-8
4. Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest. 2009;135(2):419-24. http://dx.doi.org/10.1378/chest.08-1235
5. Stănescu D, Veriter C. A normal FEV1/VC ratio does not exclude airway obstruction. Respiration. 2004;71(4):348-52. http://dx.doi.org/10.1159/000079638
6. Salome MC, King GG, Berend N. Effects of obesity on lung function. In: Dixon AE, Clerisme-Beaty EM, editors. Obesity and lung disease: a guide to management. New York: Springer Science; 2013. p. 1-20. http://dx.doi.org/10.1007/978-1-62703-053-3_1
7. Ryu JH, Scanlon PD. Obstructive lung diseases: COPD, asthma, and many imitators. Mayo Clin Proc. 2001;76(11):1144-53. http://dx.doi.org/10.4065/76.11.1144
8. Kaminsky DA. What does airway resistance tell us about lung function? Respir Care. 2012;57(1):85-96. http://dx.doi.org/10.4187/respcare.01411
9. Niewoehner DE, Kleinerman J. Morphologic basis of pulmonary resistance in the human lung and effects of aging. J Appl Physiol. 1974;36(4):412-8.
10. Bassiri AG, Girgis RE, Doyle RL, Theodore J. Detection of small airway dysfunction using specific airway conductance. Chest. 1997;111(6):1533-5. http://dx.doi.org/10.1378/chest.111.6.1533
11. Piatti G, Fasano V, Cantarella G, Tarantola C. Body plethysmographic study of specific airway resistance in a sample of healthy adults. Respirology. 2012;17(6):976-83. http://dx.doi.org/10.1111/j.1440-1843.2012.02206.x
12. Aguiar VA, Beppu OS, Romaldini H, Ratto OR, Nakatani J. Validity of a respiratory modified questionnaire (ATS-DLS-78) as a tool of an epidemiologic study in Brazil [Article in Portuguese]. J Pneumol. 1988;14(3):111-6.
13. Sociedade Brasileira de Pneumologia e Tisiologia. Diretrizes para testes de função pulmonar. J Pneumol. 2002;28(Suppl 3):S1-S238.
14. Pereira CA, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33(4):397-406. http://dx.doi.org/10.1590/S1806-37132007000400008
15. Pereira CA. Espirometria. J Pneumol. 2002;28(Suppl 3)S1-S82.
16. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107-36. http://dx.doi.org/10.1164/ajrccm.152.3.7663792
17. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511-22. http://dx.doi.org/10.1183/09031936.05.00035005
18. Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18(3):419-25.
19. Soares AL, Pereira CA, Rodrigues SC. Spirometric changes in obstructive disease: after all, how much is significant? J Bras Pneumol. 2013;39(1):56-62. http://dx.doi.org/10.1590/S1806-37132013000100008
20. Matthys H, Orth U. Comparative measurements of airway resistance. Respiration.1975;32(2):121-34. http://dx.doi.org/10.1159/000193642
21. Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state. Predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis. 1983;127(3):270-7.
22. Neder JA, Andreoni S, Castelo-Filho A, Nery LE. Reference values for lung function tests. I. Static volumes. Braz J Med Biol Res. 1999;32(6):703-17. http://dx.doi.org/10.1590/s0100-879x1999000600006
23. Miller A, Palecki A. Restrictive impairment in patients with asthma. Respir Med. 2007; 101(2):272-6. http://dx.doi.org/10.1016/j.rmed.2006.05.008
24. Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and functional limitation: data from the Third National Health and Nutrition Examination. J Intern Med. 2003;254(6),540-7. http://dx.doi.org/10.1111/j.1365-2796.2003.01211.x
25. Soriano JB, Miravitlles M, García-Río F, Muñoz L. Sánchez G, Sobradillo V, et al. Spirometrically-defined restrictive ventilatory defect: population variability and individual determinants. Prim Care Respir J. 2012;21(2):187-93. http://dx.doi.org/10.4104/pcrj.2012.00027
26. Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, et al. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184(1):57-63. http://dx.doi.org/10.1164/rccm.201101-0021OC
27. Sverzellati N, Guerci L, Randi G, Calabrò E, La Vecchia C, Marchianò A, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38(2):392-400. http://dx.doi.org/10.1183/09031936.00201809
28. Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130(3):827-33. http://dx.doi.org/10.1378/chest.130.3.827
29. Nicolacakis K, Skowronski ME, Coreno AJ, West E, Nader NZ, Smith RL, et al. Observations on the physiological interactions between obesity and asthma. J Appl Physiol (1985). 2008;105(5):1533-41. http://dx.doi.org/10.1152/japplphysiol.01260.2007
30. Brashier B, Salvi S. Obesity and asthma: physiological perspective. J Allergy (Cairo). 2013;2013:198068. http://dx.doi.org/10.1155/2013/198068
31. Brazzale DJ, Pretto JJ, Schachter LM. Optimizing respiratory function assessments to elucidate the impact of obesity on respiratory health. Respirology. 2015;20(5):715-21. http://dx.doi.org/10.1111/resp.12563
32. van Huisstede A, Castro Cabezas M, van de Geijn GJ, Mannaerts GH, Njo TL, Taube C, et al. Underdiagnosis and overdiagnosis of asthma in the morbidly obese. Respir Med. 2013;107(9):1356-64. http://dx.doi.org/10.1016/j.rmed.2013.05.007
33. Hong Y, Ra SW, Shim TS, Lim CM, Koh Y, Lee SD, et al. Poor interpretation of pulmonary function tests in patients with concomitant decreases in FEV1 and FVC. Respirology. 2008;13(4):569-74. http://dx.doi.org/10.1111/j.1440-1843.2008.01274.x
34. Gilbert R, Auchincloss JH Jr. The interpretation of the spirogram. How accurate is it for 'obstruction'? Arch Intern Med. 1985;145(9):1635-9. http://dx.doi.org/10.1001/archinte.1985.00360090103016
35. Jankowich MD, Rounds SI. Combined pulmonary fibrosis and emphysema syndrome: a review. Chest. 2012;141(1):222-31. http://dx.doi.org/10.1378/chest.11-1062
36. Cottin V, Cordier JF. Combined pulmonary fibrosis and emphysema in connective tissue disease. Curr Opin Pulm Med. 2012;18(5):418-27. http://dx.doi.org/10.1097/MCP.0b013e328356803b
37. Wells AU. Pulmonary function tests in connective tissue disease. Semin Respir Crit Care Med. 2007;28(4):379-88. http://dx.doi.org/10.1055/s-2007-985610
38. Bourke SJ, Carter R, Anderson K, Boyd J, King S, Douglas B, Boyd G. Obstructive airways disease in non-smoking subjects with pigeon fanciers' lung. Clin Exp Allergy. 1989;19(6):629-32. http://dx.doi.org/10.1111/j.1365-2222.1989.tb02758.x
39. Laohaburanakit P, Chan A. Obstructive sarcoidosis. Clin Rev Allergy Immunol. 2003;25(2):115-29. http://dx.doi.org/10.1385/CRIAI:25:2:115






