Ao Editor:Descrevemos aqui o caso de um homem de 75 anos que apresentava queixas de dispneia de esforço progressiva (grau II/III pela escala modified Medical Research Council) e tosse seca persistente ao longo dos últimos três anos. Havia trabalhado durante 35 anos como soldador profissional, realizando solda de ligas metálicas, polimento das peças soldadas com jateamento de areia e granalha de aço e prática regular de isolamento de utensílios fixos com fibras de amianto.
Orientado na esfera de cuidados primários durante o primeiro ano, veio a desenvolver lesões cutâneas descamativas cíclicas nos membros, com aspecto de liquenificação/descamação, fenômeno de Raynaud, síndrome sicca e perda ponderal de 5 kg. Ao exame físico mostrava-se eupneico, com SpO2 de 95%, apresentando crepitações inspiratórias basais à auscultação e ausência de hipocratismo digital.
A radiografia de tórax evidenciava alterações intersticiais de tipo reticular. A TC torácica demonstrou adenopatias mediastínicas calcificadas em "casca de ovo", reticulação interlobular, bronquiectasias de tração e espessamento de septos, com áreas em padrão de vidro fosco, consolidação alveolar de distribuição peribroncovascular e zonas de espessamento pleural (Figura 1).
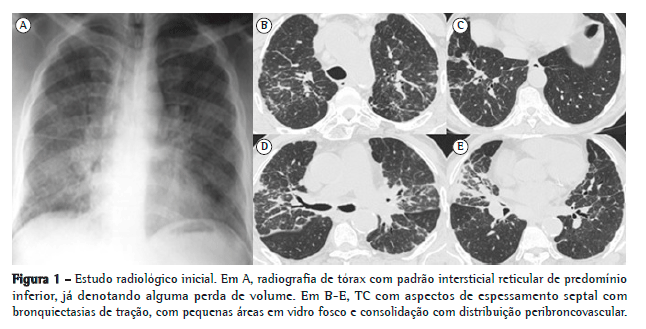
O paciente foi submetido a biópsia cutânea, que mostrou alterações de padrão liquenoide sem especificidade apurável. O lavado broncoalveolar (LBA), realizada ao nível do lobo médio (brônquio direito 4a), revelou celularidade total de 130.000 células/mL, com 40% de linfócitos e 16% de neutrófilos, com estudo microbiológico e citopatológico negativos. A imunofenotipagem mostrou predomínio de linfócitos T CD8 (razão CD4/CD8 = 0,68) e de linfócitos B (20%). A fração inorgânica não revelou corpos de asbesto e foi enviada para doseamento de metais duros e silicatos pela técnica de espectrometria de emissão atômica em plasma indutivamente acoplado. Essa pesquisa mostrou níveis elevados de sílica, cobre, cobalto, cromo, rubídio, molibdênio e zinco.
Analiticamente evidenciava hemoglobina de 11,6 g/dL, parâmetros inflamatórios normais, creatinina de 1,0 mg/dL e sedimento urinário inativo. Apresentava uma 2-microglobulina de 6,56 mg/L, enzima de conversão de angiotensina de 127 U/L, hipergamaglobulinemia policlonal IgG e IgA, e presença de anticorpos antinucleares positivos com títulos elevados de anti-SSA60 e anti-SSB. A xeroftalmia foi comprovada por teste de Schirmer (olho direito, 9 mm; olho esquerdo, 7 mm).
Funcionalmente, apresentava um padrão restritivo moderado (índice de Tiffeneau, 80; VEF1, 70,9%; CVF, 66,8%; CPT, 64,6%; e VR, 68,4%) com diminuição moderada da DLCO (51,2% do previsto) e baixa distância percorrida na prova de marcha com dessaturação de 5%.
A biópsia pulmonar cirúrgica evidenciou focos de proliferação fibroblástica, macrófagos de pigmentação antracótica e partículas birrefringentes sugestivas de silicatos, associados a células gigantes multinucleadas ao longo de eixos broncovasculares e septos interlobulares, com descamação macrofágica alveolar e fibrose pleural difusa generalizada (Figura 2).
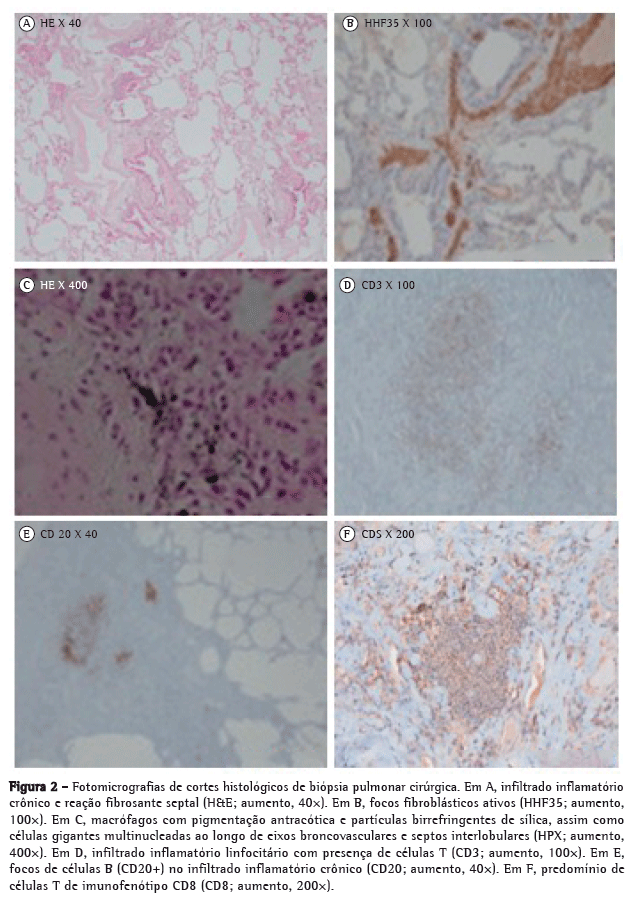
Foi firmado o diagnóstico de pneumoconiose mista por sílica, metais duros e asbesto, associada à síndrome de Sjögren (SS) primária com possível envolvimento parenquimatoso pulmonar. Iniciou tratamento com prednisolona 0,5 mg/kg em ciclo de quatro meses com melhoria da dispneia de esforço e remissão completa das lesões cutâneas, embora apenas com tênue melhoria radiológica e da DLCO.
O diagnóstico paralelo da SS primária de início tardio, cumprindo os critérios propostos pelo Grupo de Consenso Euro-Americano, pode ser relacionado com o longo período de exposição à sílica, de acordo com alguns raros casos reportados na literatura.(1-3) De fato, a intensa exposição à sílica mostrou poder conduzir ao desenvolvimento de processos autoimunes numa fração dos trabalhadores expostos, designadamente esclerose sistêmica,(4) artrite reumatoide(4) e SS primária. (1-4) Nesse contexto, a linfocitose alveolar foi já correlacionada com o envolvimento pulmonar por SS e conotada com prognóstico desfavorável.(5) No caso em consideração, o achado de infiltração linfocitária peribroncovascular com proporção relevante de linfócitos B pode relacionar-se com infiltração parenquimatosa imputável à SS. A exclusão correta de linfoma foi essencial.
O componente pneumoconiótico, expresso radiologicamente por alterações inflamatórias já com comprometimento fibrótico, é manifestado histologicamente pela reação fibroblástica perisseptal e peribroncovascular, assim como pela presença de macrófagos com pigmento antracótico e partículas birrefringentes. Os aspetos encontrados de descamação alveolar e reação de células gigantes constituem os tipicamente observados na doença pulmonar por metais duros.(6-8) As áreas de fibrose pleural difusa são relacionáveis com a exposição ao asbesto.
A histologia típica da doença parenquimatosa induzida por metais duros corresponde ao padrão de fibrose intersticial com reação de células gigantes e focos de pneumonia intersticial descamativa com ou sem bronquiolite obliterante. (6-8) Pontualmente, poderá haver uma disposição sarcoide ou somente um padrão de pneumoconiose do tipo poeira mista.
A análise mineralógica pulmonar é útil na detecção etiológica de partículas em pneumoconioses.(7) No presente caso, a pesquisa de silicatos e metais duros em LBA foi feita recorrendo a espectrometria de emissão atômica em plasma indutivamente acoplado. Esse tipo de informação permite documentar exposições ocupacionais, muitas vezes mistas, várias décadas após o seu término, auxiliando na determinação etiológica de algumas doenças respiratórias ocupacionais.(9) Apesar de a análise direta em biópsia/necropsia pulmonar constituir o indicador mais direto para estudos de acumulação de partículas, o LBA apresenta maior simplicidade e boa concordância para com os resultados obtidos de tecido.(9) A biópsia torna-se, contudo, indispensável em casos que imponham a distinção com sarcoidose.
Os metais duros de maior aplicação industrial são carbonetos de tungstênio, de molibdênio e de cromo - sendo o cobalto e o níquel elementos de ligação - e podem induzir tanto respostas imunológicas antígeno-específicas no pulmão como respostas imunes inatas caracterizadas por inflamação, frequentemente desencadeadas por lesão oxidativa.(8) Dos elementos detectados a partir de LBA do paciente, tanto a sílica como o cromo, molibdênio, cobalto e zinco foram já associados à fibrose pulmonar ou pneumoconiose. (10) Sabe-se, porém, que altas concentrações de partículas em tecidos ou fluidos orgânicos indicam exposição relevante mas não obrigatoriamente doença. No entanto, quando enquadradas em circunstâncias de forte exposição e contexto clínico, radiológico e histológico sugestivos, como no presente caso, as determinações de metais duros são um elemento valioso no diagnóstico e na compreensão patogênica de pneumoconioses menos habituais.(7)
Dado o largo contingente de trabalhadores envolvidos, a melhor compreensão das repercussões sobre a função pulmonar induzidas por exposição a fumos de soldadura será importante para o desenvolvimento de melhores estratégias de prevenção.
Pedro Gonçalo de Silva Ferreira
Pneumologista, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
António Jorge Correia Gouveia Ferreira
Pneumologista, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
Lina Maria Rodrigues de Carvalho
Diretora, Departamento de Anatomopatologia, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
António Segorbe Luís
Diretor, Departamento de Imunoalergologia, Centro Hospitalar e Universitário de Coimbra, Coimbra, PortugalReferências1. Puisieux F, Hachulla E, Brouillard M, Hatron PY, Devulder B. Silicosis and primary Gougerot-Sjögren syndrome [Article in French]. Rev Med Interne. 1994;15(9):575-9. http://dx.doi.org/10.1016/S0248-8663(05)82502-0
2. Astudillo L, Sailler L, Ecoiffier M, Giron J, Couret B, Arlet-Suau E. Exposure to silica and primary Sjögren's syndrome in a dental technician. Rheumatology (Oxford). 2003;42(10):1268-9. http://dx.doi.org/10.1093/rheumatology/keg334 PMid:14508049
3. Kirwan JR. Out-patient workload. Rheumatology (Oxford). 2003;42(10):1269-70. http://dx.doi.org/10.1093/rheumatology/keg335 PMid:14508050
4. Sanchez-Roman J, Wichmann I, Salaberri J, Varela JM, Nu-ez-Roldan A. Multiple clinical and biological autoimmune manifestations in 50 workers after occupational exposure to silica. Ann Rheum Dis. 1993;52(7):534-8. http://dx.doi.org/10.1136/ard.52.7.534 PMid:8394065 PMCid:PMC1005094
5. Dalavanga YA, Voulgari PV, Georgiadis AN, Leontaridi C, Katsenos S, Vassiliou M, et al. Lymphocytic alveolitis: A surprising index of poor prognosis in patients with primary Sjogren's syndrome. Rheumatol Int. 2006;26(9):799-804. http://dx.doi.org/10.1007/s00296-005-0092-1 PMid:16344933
6. van den Eeckhout AV, Verbeken E, Demedts M. Pulmonary pathology due to cobalt and hard metals [Article in French]. Rev Mal Respir. 1989;6(3):201-7. PMid:2662276
7. Rüttner JR, Spycher MA, Stolkin I. Inorganic particulates in pneumoconiotic lungs of hard metal grinders. Br J Ind Med. 1987;44(10):657-60. PMid:3676118 PMCid:PMC1007897
8. Kelleher P, Pacheco K, Newman LS. Inorganic dust pneumonias: the metal-related parenchymal disorders. Environ Health Perspect. 2000;108 Suppl 4:685-96. PMid:10931787 PMCid:PMC1637664
9. Dumortier P, De Vuyst P, Yernault JC. Non-fibrous inorganic particles in human bronchoalveolar lavage fluids. Scanning Microsc. 1989;3(4):1207-16; discussion 1217-8. PMid:2561220
10. Selden A, Sahle W, Johansson L, Sorenson S, Persson B. Three cases of dental technician's pneumoconiosis related to cobalt-chromium-molybdenum dust exposure. Chest. 1996;109(3):837-42. http://dx.doi.org/10.1378/chest.109.3.837 PMid:8617099





