Fabiana Stanzani, Denise de Moraes Paisani, Anderson de Oliveira, Rodrigo Caetano de Souza, João Aléssio Juliano Perfeito, Sonia Maria Faresin
ABSTRACT
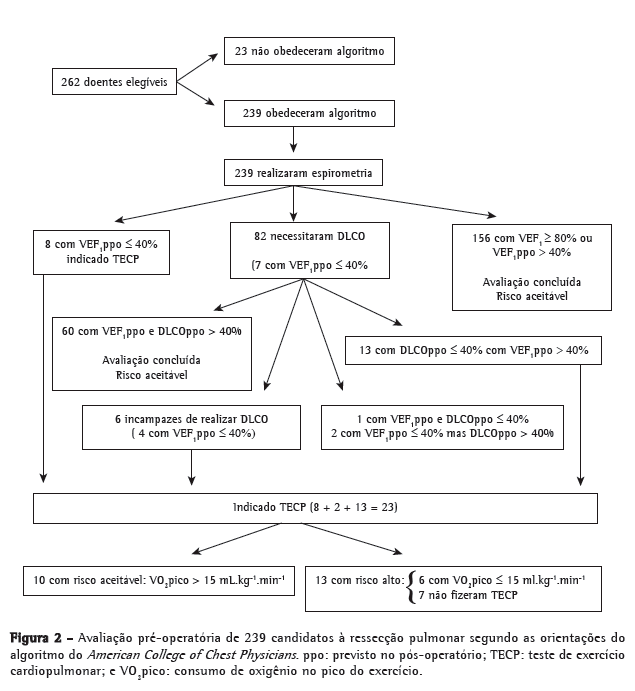
Objective: To determine morbidity and mortality rates by risk category in accordance with the American College of Chest Physicians guidelines, to determine what role pulmonary function tests play in this categorization process, and to identify risk factors for perioperative complications (PCs). Methods: This was a historical cohort study based on preoperative and postoperative data collected for cases of lung cancer diagnosed or suspected between 2001 and 2010. Results: Of the 239 patients evaluated, only 13 (5.4%) were classified as being at high risk of PCs. Predicted postoperative FEV1 (FEV1ppo) was sufficient to define the risk level in 156 patients (65.3%); however, cardiopulmonary exercise testing (CPET) was necessary for identifying those at high risk. Lung resection was performed in 145 patients. Overall morbidity and mortality rates were similar to those reported in other studies. However, morbidity and mortality rates for patients at an acceptable risk of PCs were 31.6% and 4.3%, respectively, whereas those for patients at high risk were 83.3% and 33.3%. Advanced age, COPD, lobe resection, and lower FEV1ppo were correlated with PCs. Conclusions: Although spirometry was sufficient for risk assessment in the majority of the population studied, CPET played a key role in the identification of high-risk patients, among whom the mortality rate was seven times higher than was that observed for those at an acceptable risk of PCs. The risk factors related to PCs coincided with those reported in previous studies.
Keywords: Algorithms; Lung neoplasms; Postoperative complications.
RESUMO
Objetivo: Determinar as taxas de morbidade e mortalidade por categoria de risco conforme as diretrizes do American College of Chest Physicians, verificar como exames funcionais participaram dessa categorização e identificar fatores de risco para complicações perioperatórias (CPOs). Métodos: Estudo de coorte histórica a partir de avaliações pré e pós-operatórias de casos diagnosticados ou suspeitos de câncer de pulmão avaliados entre 2001 e 2010. Resultados: Dos 239 pacientes avaliados, apenas 13 (5,4%) foram considerados como de alto risco para CPOs. O cálculo do VEF1 previsto para o pós-operatório (VEF1ppo) foi suficiente para a estratificação do risco em 156 pacientes (65,3%); entretanto, o teste de exercício cardiopulmonar (TECP) foi necessário para a identificação de alto risco. Foram operados 145 pacientes, e as taxas globais de morbidade e mortalidade encontradas foram semelhantes às de outros estudos. Entretanto, as taxas de morbidade e mortalidade para aqueles com risco aceitável foram de 31,6% e 4,3%, respectivamente, enquanto as taxas para aqueles com alto risco foram de 83,3% e 33,3%. Idade mais avançada, presença da DPOC, ressecção de um ou mais lobos e VEF1ppo mais baixo estiveram relacionados à ocorrência de CPOs. Conclusões: Embora a espirometria tenha sido suficiente para a determinação de risco na maioria da população estudada, o TECP teve papel fundamental na identificação de pacientes com risco alto, que apresentaram uma taxa de mortalidade sete vezes maior que os de risco aceitável. Os fatores de risco relacionados a CPOs coincidiram aos relatados em outros estudos.
Palavras-chave: Algoritmos; Neoplasias pulmonares; Complicações pós-operatórias.
Introdução