ABSTRACT
Objective: To analyze clinical and radiological findings that influence the pathological diagnosis of solitary pulmonary nodule (SPN) and to compare/validate two probabilistic models for predicting SPN malignancy in patients with SPN in Brazil. Methods: This was a retrospective study involving 110 patients diagnosed with SPN and submitted to resection of SPN at a tertiary hospital between 2000 and 2009. The clinical characteristics studied were gender, age, presence of systemic comorbidities, history of malignancy prior to the diagnosis of SPN, histopathological diagnosis of SPN, smoking status, smoking history, and time since smoking cessation. The radiological characteristics studied, in relation to the SPN, were presence of spiculated margins, maximum transverse diameter, and anatomical location. Two mathematical models, created in 1997 and 2007, respectively, were used in order to determine the probability of SPN malignancy. Results: We found that SPN malignancy was significantly associated with age (p = 0.006; OR = 5.70 for age > 70 years), spiculated margins (p = 0.001), and maximum diameter of SPN (p = 0.001; OR = 2.62 for diameters > 20 mm). The probabilistic model created in 1997 proved to be superior to that created in 2007-area under the ROC curve, 0.79 ± 0.44 (95% CI: 0.70 0.88) vs. 0.69 ± 0.50 (95% CI: 0.59-0.79). Conclusions: Advanced age, greater maximum SPN diameter, and spiculated margins were significantly associated with the diagnosis of SPN malignancy. Our analysis shows that, although both mathematical models were effective in determining SPN malignancy in our population, the 1997 model was superior.
Keywords:
Solitary Pulmonary Nodule; Risk Factors; Carcinoma, Non-Small-Cell Lung.
RESUMO
Objetivo: Analisar características clínicas e radiográficas que influenciaram o diagnóstico anatomopatológico de nódulo pulmonar solitário (NPS) e comparar/validar dois modelos probabilísticos de malignidade do NPS em pacientes com NPS no Brasil. Métodos: Análise retrospectiva de 110 pacientes com diagnóstico de NPS submetidos à ressecção em um hospital terciário no período entre 2000 e 2009. As características clínicas estudadas foram gênero, idade, presença de comorbidades sistêmicas, história de neoplasia maligna ao diagnóstico de NPS, diagnóstico histopatológico do NPS, tabagismo, carga tabágica e tempo de cessação do tabagismo. As características radiográficas avaliadas em relação ao NPS foram presença de margens espiculadas, tamanho do maior diâmetro transversal e localização anatômica do NPS. Foram utilizados dois modelos matemáticos, criados em 1997 e 2007, respectivamente, para determinar a probabilidade de malignidade do NPS. Resultados: Houve associações significantes entre malignidade do NPS e idade (p = 0,006; OR = 5,70 para idade >70 anos), presença de margens espiculadas (p = 0,001) e diâmetro maior do NPS (p = 0,001; OR = 2,62 para diâmetro >20 mm). O modelo probabilístico de 1997 mostrou-se superior ao de 2007 - área sob a curva [ASC] ROC = 0,79 ± 0,44 (IC95%: 0,70-0,88) vs. ASC = 0,69 ± 0,50 (IC95%: 0,59-0,79). Conclusões: Idade elevada, maior diâmetro do NPS e presença de margens espiculadas tiveram associações significantes ao diagnóstico de malignidade do NPS. Nossa análise mostrou que, embora os dois modelos matemáticos sejam eficazes na determinação de malignidade do NPS nessa população, o modelo de 1997 mostrou-se superior.
Palavras-chave:
Nódulo pulmonar solitário; Fatores de risco; Carcinoma pulmonar de células não pequenas.
IntroduçãoOs nódulos pulmonares sempre se constituíram em um grande desafio diagnóstico, preocupação justificada pela incidência de neoplasias malignas pulmonares, sejam elas metastáticas, sejam primárias. Nas últimas décadas, observamos um aumento da incidência e, consequentemente, da mortalidade, causado pela neoplasia primária pulmonar, concomitante à melhoria das técnicas de imagem, o que causou uma maior detecção dos nódulos pulmonares. Nesse contexto, o achado de um nódulo pulmonar solitário (NPS) tem se tornado o ponto chave para a detecção precoce dessa neoplasia que, segundo dados do Sistema de Informações sobre Mortalidade do Ministério da Saúde brasileiro, já ocupa o primeiro lugar como causador de mortes entre as neoplasias malignas, suplantando os óbitos causados pelas neoplasias de próstata e mama, quando não consideramos o sexo para o diagnóstico.
O NPS é definido como uma opacidade pulmonar mais ou menos esférica com diâmetro menor que três centímetros. Geralmente tem contornos identificáveis, totalmente circundados por parênquima pulmonar e sem outras alterações radiográficas, como atelectasias ou aumento de linfonodos mediastinais.(1,2)
Diversas formas de estimar o potencial de malignidade do NPS foram criadas, e entre as mais divulgadas estão os modelos matemáticos baseados em análise multivariada das características clínicas dos pacientes portadores de NPS e as características radiológicas desses nódulos, publicados primeiramente por Swensen et al.(3) em 1997 e, posteriormente, por Gould et al. em 2007.(4) Nos dois estudos, foram criadas fórmulas matemáticas para o cálculo da probabilidade de malignidade do NPS a fim de guiar a conduta do médico assistente, e os modelos foram amplamente testados e aprovados principalmente em populações nos EUA e Europa.(3-5) Em uma avaliação em pacientes das Filipinas, a alta prevalência de tuberculose não permitiu que se repetisse tal achado, demonstrando a inefetividade do método para aquela população.(6)
Frente à inobservância, até o momento, de trabalhos nacionais publicados com o intuito de avaliar tais modelos em nossa população, o objetivo do presente estudo foi analisar variáveis clínicas e radiográficas que interferiram no diagnóstico anatomopatológico do NPS e, dessa forma, validar e comparar os dois modelos matemáticos desenvolvidos nos estudos previamente citados(3,4) para o cálculo da probabilidade de malignidade frente ao diagnóstico de NPS.
MétodosEstudo retrospectivo de todos os pacientes submetidos a ressecção de NPS no Hospital São Paulo, na cidade de São Paulo (SP) no período entre 2000 e 2009 com base em dados dos prontuários médicos. Foram estudadas as seguintes variáveis: gênero, idade, presença de comorbidades sistêmicas, presença de neoplasia maligna prévia ao diagnóstico do nódulo, diagnóstico histopatológico do mesmo (doença maligna vs. doença benigna), histórico de tabagismo (pacientes com relato de consumo atual e aqueles que descontinuaram o consumo), carga tabágica em maços-ano e quantidade de anos após a cessação do tabagismo. Foram também estudadas características tomográficas do NPS, tais como presença de contornos espiculados, tamanho do maior diâmetro transversal (em mm) e localização anatômica pulmonar, baseadas em laudos de exame.
Após a coleta dos dados, obedecemos aos seguintes critérios de inclusão: diagnóstico firmado de NPS, pacientes submetidos à ressecção cirúrgica do NPS, presença de dados a serem estudados no prontuário e diagnóstico anatomopatológico do NPS.
Arrolamos, então, 127 pacientes que, após a aplicação dos critérios acima, totalizaram 110 indivíduos. Daqueles excluídos do estudo, a maioria o foi por ausência de dados anotados nos prontuários, seguida por diagnóstico de múltiplos nódulos pulmonares.
Definimos a probabilidade de malignidade do NPS através dos modelos matemáticos desenvolvidos previamente naqueles estudos de acordo com as equações matemáticas definidas por eles:
Equações segundo Swensen et al.(3):
Probabilidade de malignidade = ex/(1+ ex) (1)
x = −6,8272 + (0,0391 × I) +
+(0,7917 × T) + (1,3388 × N) +
+(0,1274 × D) + (1,0407 × E) +
+(0,7838 × LS) (2)
onde I é a idade em anos, T é a presença ou ausência de tabagismo (0 para ausência e 1 para presença), N é a presença ou ausência de neoplasia prévia (0 para ausência e 1 para presença), D é o diâmetro em mm, E é a presença ou ausência de margens espiculadas (0 para ausência e 1 para presença) e LS é a localização anatômica (1 para presença em lobos superiores e 0 em outros locais).
Equações segundo Gould et al.(4):
Probabilidade de malignidade = ex/(1+ ex) (1)
x = −8,404 + (0,779 × I) + (2,061 × T) + + (0,112 × D) − (0,567 × A) (3)
onde I é a idade em anos, T é a presença ou ausência de tabagismo (0 para ausência e 1 para presença), D é o diâmetro em mm e A é o número de anos sem tabagismo dividido por 10.
Para a análise estatística, utilizamos o programa Statistical Package for the Social Sciences for Windows, versão 13.0, (SPSS Inc., Chicago, IL, EUA) e sua versão para Mac, versão 20.0. Utilizamos também o programa BioEstat, versão 5.0 para Windows, para análises complementares e construção das curvas ROC.
Para as variáveis paramétricas, foi aplicado o teste t de Student; para as variáveis não paramétricas, aplicamos o teste do qui-quadrado de Pearson; e, para variáveis dicotômicas, utilizamos o teste exato de Fisher, com o intuito de verificarmos possíveis diferenças entre os grupos estudados.(7) Adotamos o nível de significância de 5% para a aplicação dos testes estatísticos.
A determinação do desempenho diagnóstico e a obtenção do melhor ponto de corte para ambos os modelos matemáticos foi realizada pela análise das curvas ROC e two-graph (TG)-ROC.(8) A sensibilidade, a especificidade, a acurácia e os valores preditivos negativos e positivos dos testes puderam ser calculados para os pontos de corte, assim como a área sob a curva e a comparação entre os métodos.
ResultadosForam avaliados 110 pacientes, 59 homens e 51 mulheres, dos quais não se observou uma associação significante ao diagnóstico de neoplasia maligna do NPS. O mesmo achado verificou-se quando se analisou a presença de comorbidades, a presença de neoplasia maligna prévia ao diagnóstico do NPS e o histórico de tabagismo. A quantidade de anos sem fumo após a cessação também não interferiu no diagnóstico anatomopatológico do NPS, assim como a média de consumo em maços-ano. A característica clínica associada significativamente foi idade (p = 0,006), quando estratificada em grupos etários, com OR crescentes, culminando em OR = 5,70 para o grupo > 70 anos (Tabela 1).
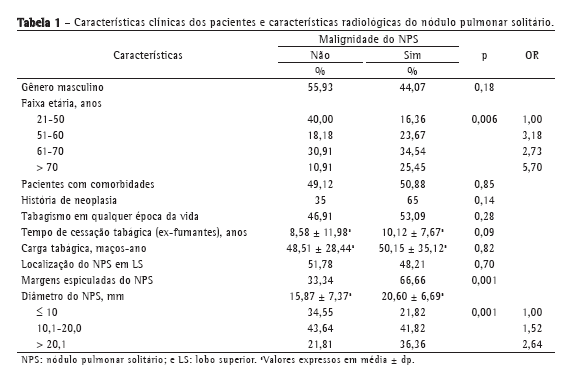
Entre as características radiológicas do NPS, a presença de margens espiculadas e o diâmetro da lesão demonstraram associações significantes (p = 0,001 para ambos). Estratificando a análise do diâmetro, encontramos OR crescentes para os extratos, chegando a OR = 2,64 naqueles NPS com diâmetro entre 20,1 mm e 30 mm (Tabela 1).
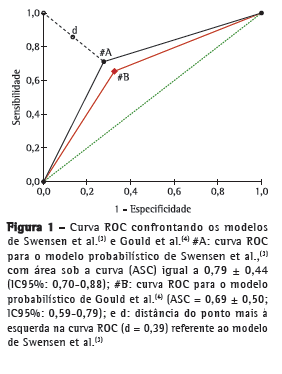
Após o cálculo das probabilidades pelo modelo matemático de Swensen et al.,(3) traçamos a curva ROC, cuja área sob a curva (ASC) foi de 0,79 ± 0,44 (IC95%: 0,70-0,88; Figura 1). A construção da curva TG-ROC nos permitiu estimar um ponto de corte ótimo frente aos diversos pontos oriundos da curva ROC (Figura 2), obtendo-se maior rendimento da sensibilidade e especificidade abaixo de 15,0% ou acima de 66,5% (rendimento maior que 95%; Tabela 2, Figura 2).
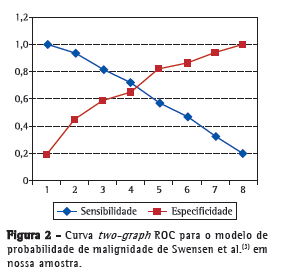
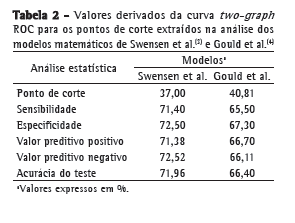
Para o modelo de Gould et al.,(4) obtivemos uma ASC de 0,69 ± 0,50 (IC95%: 0,59-0,79; Figura 3). Através da análise da curva TG-ROC, observamos o comportamento dos diversos pontos de corte frente a sensibilidade e especificidade e, para um rendimento máximo (maior que 95%), os pontos de corte calculados se encontram abaixo de 8,5% e acima de 82,3% (Tabela 2, Figura 3).
 Discussão
DiscussãoO diagnóstico do NPS ainda permanece como um grande desafio na prática médica. No presente estudo, avaliamos uma casuística de pacientes submetidos a ressecção cirúrgica do NPS com o intuito de definir o diagnóstico anatomopatológico e identificamos três fatores de risco independentes: idade avançada, presença de margens espiculadas do NPS e diâmetro do NPS. As demais características clínicas e radiográficas dos pacientes com NPS não demonstraram associações significantes em nossa casuística.
A idade vem sendo apontada, em diversos estudos recentes, como um dos principais fatores de risco para o diagnóstico de malignidade no NPS.(3,4,9,10) Verificamos uma associação estatisticamente significante e OR crescentes através das estratificações etárias, corroborando os achados atuais, que demonstram que as faixas etárias mais altas, principalmente acima dos 50 anos, estão em maior risco de diagnóstico de NPS maligno.
Margens espiculadas do tipo corona radiata predizem a natureza maligna do NPS, com valor preditivo positivo chegando a 94%, enquanto margens lobuladas predizem malignidade em até 80%.(11-13) Tal comportamento também foi observado no presente estudo, pelo qual evidenciamos que dois terços das lesões malignas tinham bordas irregulares espiculadas ou lobuladas, com significância estatística (p = 0,001).
O diâmetro médio da lesão também é um importante fator de risco para a malignidade, especialmente quando esse aumenta e se aproxima de 30 mm. Inúmeros estudos comprovaram tal achado, sempre associando o crescimento da lesão ao seu potencial de malignidade. Nódulos com diâmetro superior a 20 mm tem mais de 50% de chance de um diagnóstico de neoplasia maligna.(14,15) Observamos o mesmo comportamento em nossos dados, com associação significante quando comparamos as médias dos diâmetros entre os grupos, sendo encontrados OR crescentes na estratificação aplicada.
Avaliamos também dois modelos matemáticos de previsão da chance de malignidade em NPS, amplamente divulgados, mas que, até o momento, não encontramos referências de estudos em nossa população. Recentemente, um grupo filipino, ao testar o modelo de Swensen et al.(3) em sua população, não verificou sua validade como preditor de malignidade, fato associado ao alto índice de tuberculose na população envolvida.(6) Em nossa população, observamos que ambos os modelos se mostraram eficazes em predizer o potencial de malignidade do NPS, sendo que o modelo de Swensen et al.(3) apresentou-se mais preciso que o de Gould et al.(4)
Analisando nossos resultados com a utilização das equações de Swensen et al.,(3) a ASC obtida no presente estudo (0,79 ± 0,44; IC95%: 0,70 0,88) foi quase idêntica aos valores obtidos por outros grupos de pesquisadores que validaram ambos os modelos probabilísticos em pesquisas semelhantes.(16,17) Tal ASC nos permite afirmar que o modelo matemático acima apresentou boa acurácia (ASC > 0,70), corroborando seu uso como um teste diagnóstico, conforme já proposto.(18) Analisando as curvas ROC e TG-ROC, observamos o comportamento através dos diversos pontos de corte: as medidas situadas nas extremidades do gráfico, isto é, os pontos de corte abaixo de 15,0% e acima de 66,5%, são os que apresentam melhor rendimento, com sensibilidade de 95,9% e especificidade de 35,3% para os pontos de corte abaixo de 15,0%, e com sensibilidade de 36,7% e especificidade de 94,1% para os pontos de corte acima de 66,5%. Em suma, pacientes com probabilidade de malignidade 15,0% possuem taxas tão elevadas de verdadeiros positivos (elevada sensibilidade) que, em tese, nos autorizariam a optar por uma conduta expectante em nossa amostra, ao passo que pacientes com probabilidade 11,0% apresentaram sensibilidade de 100%, sendo essa, portanto, a menor taxa possível de exames falso-negativos. Na outra ponta da curva, encontramos os pacientes com probabilidade de malignidade 66,5%; neste ponto de corte, verificou-se sensibilidade de 36,7% e especificidade de 94,1%, ou seja, atingindo valores que autorizam a indicar a ressecção cirúrgica do NPS frente a um alto índice diagnóstico, que aumenta após aquele percentil, chegando a 100% de especificidade após o ponto de corte de 80,5% (isto é, reduzindo ao máximo a figura de falso-negativos). Para os pacientes com probabilidade intermediária (ou seja, aqueles cujo cálculo de probabilidade se situou entre 15,0% e 66,5%), o modelo não se mostrou eficaz em predizer a probabilidade de malignidade do nódulo, não servindo, portanto, como um teste diagnóstico confiável. Para esses pacientes, maiores investigações são necessárias, como o uso de tomografia de emissão de prótons ou algum tipo de biópsia (transtorácica ou transbrônquica).
No modelo de Gould et al.,(4) a análise da curva ROC demonstrou uma ASC de 0,69 ± 0,50 (IC95%: 0,59-0,79) e acurácia de 66,40%. Só obtivemos pontos de corte confiáveis com valores 8,36% (sensibilidade de 94,4% e especificidade de 21,4%) e valores 82,3% (sensibilidade de 13,0% e especificidade de 94,6%). Portanto, para os casos dentro do intervalo entre esses pontos de corte, não é póssível chegar a conclusões confiáveis através do método, sendo necessárias outras investigações.
Para nossa amostra, verificamos a superioridade do modelo matemático de Swensen et al.(3) frente ao modelo de Gould et al.(4); através da análise da sobreposição das curvas ROC; observamos uma ASC maior para o primeiro método, além de uma menor amplitude do intervalo entre os pontos de corte confiáveis. Esse comportamento demonstrou, em nossa casuística, uma maior acurácia diagnóstica do modelo matemático proposto por Swensen et al.(3) Recentemente, em um estudo realizado nos EUA, os dois modelos foram comparados, sendo identificados comportamentos bastante semelhantes entre esses métodos,(16) diferentemente da nossa observação.
Concluindo, dentre as características clínicas e radiográficas relacionadas ao NPS, três delas demonstraram significância estatística quando associadas à malignidade do NPS: idade mais avançada do paciente, presença de margens espiculadas do NPS na TC de tórax e diâmetro maior do NPS.
A análise comparativa entre os métodos demonstrou que o modelo de Swensen et al.,(3) em comparação ao de Gould et al.,(4) apresentou melhor rendimento, com sensibilidade, especificidade e acurácia maiores.
Referências1. Varoli F, Vergani C, Caminiti R, Francese M, Gerosa C, Bongini M, et al. Management of solitary pulmonary nodule. Eur J Cardiothorac Surg. 2008;33(3):461-5. PMid:18203611. http://dx.doi.org/10.1016/j.ejcts.2007.12.004
2. Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med. 2003;348(25):2535-42. PMid:12815140. http://dx.doi.org/10.1056/NEJMcp012290
3. Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849 55. PMid:9129544. http://dx.doi.org/10.1001/archinte.1997.00440290031002
4. Gould MK, Ananth L, Barnett PG; Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131(2):383-8. PMid:17296637 PMCid:3008547. http://dx.doi.org/10.1378/chest.06-1261
5. Schultz EM, Sanders GD, Trotter PR, Patz EF Jr, Silvestri GA, Owens DK, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335 41. PMid:17965070 PMCid:2882437. http://dx.doi.org/10.1136/thx.2007.084731
6. Rafanan AL, Ceniza SV, Canete MT. Two commonly used prediction models (Mayo and VA) to estimate the probability of malignancy in patients with solitary pulmonary nodules are not applicable in a country with a high prevalence of tuberculosis. Chest. 2010;138(4_MeetingAbstracts):250A-250A. doi:10.1378/chest.10657
7. Ebraim GJ, Sullivan KR. Mother and Child Health Research Methods. London: Book-Aid; 1995.
8. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29-36. PMid:7063747.
9. Gould MK, Fletcher J, Iannettoni MD, Lynch WR, Midthun DE, Naidich DP, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):108S-130S.
10. Clements WM, DeRosimo JF, Reed CE. Solitary pulmonary nodule. In: Shields TW, LoCicero J, Reed CE, Feins RH, editors. General Thoracic Surgery. Philadelphia: Lippincott Williams Wilkins; 2009. p. 1205-11.
11. Soubani AO. The evaluation and management of the solitary pulmonary nodule. Postgrad Med J. 2008;84(995):459 66. PMid:18940947. http://dx.doi.org/10.1136/pgmj.2007.063545
12. Stark P. Computed tomographic and positron emission tomographic scanning of pulmonary nodules. In: UpToDate, Basow DS, editor, UpToDate: Waltham, MA; 2012.
13. Seemann MD, Seemann O, Luboldt W, Bonél H, Sittek H, Dienemann H, et al. Differentiation of malignant from benign solitary pulmonary lesions using chest radiography, spiral CT and HRCT. Lung Cancer. 2000;29(2):105-24. http://dx.doi.org/10.1016/S0169-5002(00)00104-5
14. Henschke CI, Yankelevitz DF, Naidich DP, McCauley DI, McGuinness G, Libby DM, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology. 2004;231(1):164-8. PMid:14990809. http://dx.doi.org/10.1148/radiol.2311030634
15. MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. PMid:16244247. http://dx.doi.org/10.1148/radiol.2372041887
16. Schultz EM, Sanders GD, Trotter PR, Patz EF Jr, Silvestri GA, Owens DK, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-41. PMid:17965070 PMCid:2882437. http://dx.doi.org/10.1136/thx.2007.084731
17. Herder GJ, van Tinteren H, Golding RP, Kostense PJ, Comans EF, Smit EF, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest. 2005;128(4):2490-6. PMid:16236914. http://dx.doi.org/10.1378/chest.128.4.2490
18. Martinez EZ, Louzada-Neto F, Pereira BB. A curva ROC para testes diagnósticos. Cad Saude Coletiva (Rio J.). 2003;11(1):7-31.
Trabalho realizado na Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
Endereço para correspondência: Cromwell Barbosa de Carvalho Melo. Rua Napoleão de Barros, 715, 4º andar, Disciplina de Cirurgia Torácica. Vila Clementino, CEP 04023-002, São Paulo, SP, Brasil.
Tel. 55 11 5576-4295. E-mail: cromwellmelo@hotmail.com
Apoio financeiro: Nenhum.
Recebido para publicação em 15/5/2012. Aprovado, após revisão, em 14/8/2012.
Sobre os autoresCromwell Barbosa de Carvalho Melo
Médico Cirurgião Torácico. Hospital São Paulo, Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
João Aléssio Juliano Perfeito
Professor Adjunto. Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
Danilo Félix Daud
Médico Cirurgião Torácico. Hospital São Paulo, Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
Altair da Silva Costa Júnior
Médico Assistente. Hospital São Paulo, Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
Ilka Lopes Santoro
Professora Afiliada. Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
Luiz Eduardo Villaça Leão
Professor Titular. Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.






