ABSTRACT
Objective: Although lean body mass (LBM) has been associated with mortality in patients with COPD, its influence on functional limitation is not clear. The objective of this study was to analyze the cardiopulmonary variables in COPD patients with or without LBM depletion, prior to and after the six-minute walk test (6MWT). Methods: We evaluated COPD patients, 32 with LBM depletion and 36 without. All patients underwent clinical evaluation, spirometry, evaluation of body mass composition and 6MWT, as well as completing questionnaires related to quality of life and perception of dyspnea. Results: No significant differences in the severity of airway obstruction, perception of dyspnea and quality of life scores were found between the groups. The distance covered on the 6MWT was similar in COPD patients with and without LBM depletion (470.3 ± 68.5 m vs. 448.2 ± 89.2 m). However, patients with LBM depletion presented significantly greater differences between baseline and final values in terms of heart rate and Borg scale index for lower limb fatigue. There was a significant positive correlation between distance covered on the 6MWT and FEV1 (r = 0.381, p = 0.01).Conclusions: In the patients studied, functional exercise tolerance and quality of life were unaffected by LBM depletion. However, the patients with LBM depletion presented more pronounced lower limb fatigue during the 6MWT, which underscores the importance of the evaluation and treatment of systemic manifestations in COPD patients.
Keywords:
Body mass index; Pulmonary disease, chronic obstructive; Exercise tolerance.
RESUMO
Objetivo: A massa magra corporal (MMC) tem sido associada à mortalidade em pacientes com DPOC, mas seu impacto na limitação funcional é pouco conhecido. O objetivo deste trabalho foi analisar as variáveis cardiopulmonares em pacientes com DPOC, com ou sem depleção da MMC, antes e após a realização do teste de caminhada de seis minutos (TC6). Métodos: Foram avaliados pacientes com DPOC, 36 sem depleção de MMC e 32 com depleção de MMC. Todos os pacientes foram submetidos à avaliação clínica, espirometria, avaliação da composição da massa corpórea e TC6 e responderam a questionários de qualidade de vida e de percepção de dispnéia. Resultados: Não foram observadas diferenças significativas na gravidade de obstrução das vias aéreas, na percepção da dispnéia e na qualidade de vida entre os grupos. A distância percorrida no TC6 foi similar nos pacientes com DPOC com e sem depleção de MMC (470,3 ± 68,5 m vs. 448,2 ± 89,2 m). Entretanto, durante a realização do teste, os pacientes com depleção de MMC apresentaram aumento significativamente maior na diferença entre os valores final e basal da frequência cardíaca e do índice da escala de Borg para cansaço dos membros inferiores. A distância percorrida no TC6 apresentou correlação significativa positiva com o VEF1 (r = 0,381; p = 0,01). Conclusões: Não houve influência da depleção da MMC na capacidade funcional de exercício e na qualidade de vida dos pacientes estudados. Entretanto, os pacientes com depleção de MMC apresentam sintomas de fadiga dos membros inferiores mais acentuados durante o TC6, o que reforça a importância da avaliação e tratamento das manifestações sistêmicas da DPOC.
Palavras-chave:
Índice de massa corporal; Doença pulmonar obstrutiva crônica; Tolerância ao exercício.
IntroductionWorldwide, COPD is one of the leading causes of death and physical incapacity.(1) Decreased exercise tolerance limits the performance of activities of daily living and is associated with lower survival in COPD patients.(2) Various etiologies, including sedentary lifestyle, hypoxemia, hypercapnia, electrolyte disturbances, oxidative stress, systemic inflammation and peripheral muscle depletion or dysfunction, have been proposed to explain the physical incapacity of such patients.(2,3)
Although lean body mass (LBM) has been associated with mortality in patients with COPD, its influence on functional exercise limitation is not clear.(4) Some studies have found associations between LBM-related attributes and maximal oxygen uptake (VO2max).(5-7) However, few studies have shown associations between LBM and the distance that patients with COPD are able to walk in a single session.(8,9) In those studies, LBM, MIP and PaO2 were found to explain 60% of the variation in the distance covered in 12 min, and there was a significant positive correlation between LBM and the six-minute walk test (6MWT) results.(8,9)
In addition, in the national and international literature, there are no studies evaluating the differences between COPD patients with LBM depletion and those without in terms of cardiopulmonary events during the 6MWT.
Therefore, we raised the hypothesis that COPD patients with LBM depletion present more significant alterations in cardiopulmonary variables after the 6MWT than do patients without LBM depletion. The objective of the present study was to analyze heart rate, respiratory rate, oxygen saturation (determined using pulse oxymetry) and Borg scale scores (for dyspnea and lower limb fatigue) in COPD patients with and without LBM depletion, prior to and after the 6MWT.
MethodsThis was a cross-sectional study involving 68 patients with stable COPD treated at the Pulmonology Clinic of the Botucatu School of Medicine of the Universidade Estadual Paulista (UNESP, São Paulo State University). The diagnosis of COPD was made based on clinical history (including exposure to risk factors), physical examination and X-rays, the diagnostic confirmation being based on a spirometry finding of postbronchodilator airway obstruction (FEV1/FVC < 70%).(1) Clinically unstable patients, characterized by the incidence of exacerbations within the preceding three months, signs of water retention, cardiovascular diseases (except cor pulmonale) or osteoarticular diseases, were excluded from the study.
The study protocol was approved by the Ethics in Research Committee of the UNESP Botucatu School of Medicine Hospital das Clínicas. All patients were informed of the procedures proposed, after which all gave written informed consent.
Spirometry was performed using a computer-assisted system of pulmonary function assessment (Koko; Ferraris Respiratory, Louisville, CO, USA), in accordance with the American Thoracic Society criteria.(10) The FEV1, FVC and FEV1/FVC ratio values were determined prior to and after the administration of 400 µg of inhaled fenoterol. The FEV1 value is expressed in liters, in percentage of FVC and in percentage of reference values.(11)
All patients performed the 6MWT twice, the second time at least 30 min after the first, in order to minimize the learning effect. The 6MWT consisted of patients walking at their fastest pace for 6 min over a 30-m course clearly marked in a corridor. Patients were given verbal encouragement through the use of standardized phrases once a minute. Although patients could stop during the test, they were instructed to start walking again as soon as they felt ready. After 6 min, the distance covered was measured, in meters, and the greatest value obtained was registered.(12) Heart rate, respiratory rate, systolic blood pressure and diastolic blood pressure, as well as perception of dyspnea and lower limb fatigue according to the Borg scale score, were measured prior to and after the 6MWT. The SpO2 was measured prior to, during and after the test. Patients who presented an initial SpO2 < 88% received oxygen supplementation through a catheter; after 30 min of supplementation, SpO2 was reevaluated, and the test was restarted.(12)
Body composition was evaluated by anthropometry and bioelectrical impedance. Weight was measured with patients wearing light clothing and no shoes. Height was measured with patients (barefoot) standing erect against the marker, with both feet together and looking straight ahead. The reading was performed with a precision of 0.5 cm, with the bar of the vertical measuring rod touching the head of the patient. Body mass index (BMI) was calculated on the basis of weight and height [weight (kg) / height (m)2].(14) Resistance was measured by bioelectrical impedance (BIA101; RJL Systems, Detroit, MI, USA). Measurements were carried out with patients in the supine position, on the right side of the body, using four surface electrodes placed on the fist and near the ankle, in accordance with the criteria of the European Society for Clinical Nutrition and Metabolism.(15) An equation, devised according to the model developed for patients with respiratory insufficiency,(16) was used in order to estimate LBM, and the LBM index (LBMI) was determined by dividing the estimated LBM by patient height in meters squared.(15) LBM depletion was defined as an LBMI < 15 kg/m2 for females and < 16 kg/m2 for males.(17)
The instrument used to evaluate quality of life was a version of the Saint George's Respiratory Questionnaire (SGRQ), translated and validated for use in Brazil.(18) Quality of life was quantified by the total SGRQ score and the scores for the three SGRQ domains (symptoms, impact and activities). A version of the Airways Questionnaire 20 (AQ20), translated and validated for use in Brazil,(19) was also administered.
Dyspnea was evaluated using the baseline dyspnea index (BDI)(20) and the Modified Medical Research Council (MMRC) scale,(21) both translated and validated for use in Brazil.
Statistical analysisThe Student's t-test was used to compare variables with normal distribution, whereas the Mann-Whitney test was used to compare nonparametric variables. Pearson and Spearman correlation tests were used for parametric and nonparametric variables, respectively. Values of p < 0.05 were considered statistically significant. The analyses were performed using SigmaStat 3.2 (SPSS Inc., Chicago, IL, USA) and Systat 10.2 (Systat Software Inc., San Jose, CA, USA).
ResultsThe general characteristics of the patients studied according to the nutritional evaluation are presented in Table 1. We studied a total of 68 patients, 72% of whom were male. Of the 68 patients studied, 15 (22%) presented mild COPD, 23 (34%) presented moderate COPD, and 30 (22%) presented severe or extremely severe COPD. The patients with LBM depletion were similar to those without LBM depletion in terms of age, and there was no significant difference between the two groups in terms of gender or the different classifications of the severity of COPD. As expected, the patients with LBM depletion presented lower BMI and LBMI. The LBMI did not correlate with the BDI or the MMRC scale score, nor did it correlate with the AQ20 score, the total SGRQ score or any of the three SGRQ domain scores.
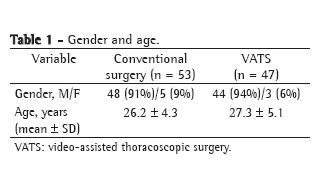
There was no significant difference between the groups in terms of the six-minute walk distance (6MWD). In the sample as a whole, the study of the correlations showed a significant positive association between the 6MWD and FEV1 (%) (r = 0.381; p = 0.01). However, the 6MWD did not present a significant association with LBMI, BMI, baseline dyspnea sensation (MMRC and BDI) or quality of life (SGRQ and AQ20).
The difference between the patients with LBM depletion and those without in terms of the change at the end of the test in relation to the baseline value (Δ) was statistically significant for heart rate (p = 0.01; Table 2). When the patients with LBM depletion were compared with the nondepleted patients in terms of Δ respiratory rate (p = 0,743), SpO2 (p = 0.946), systolic blood pressure (p = 0.313) and diastolic blood pressure (p = 0.567), no significant differences were found, nor were these variables found to correlate with LBMI or BMI.
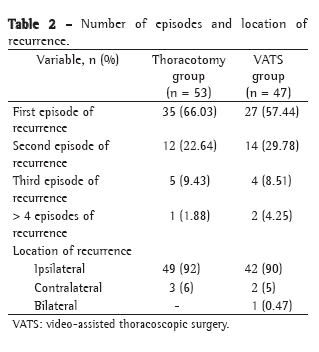
The Δ of the Borg scale score for lower limb fatigue was significantly higher in the patients with LBM depletion (p = 0.02; Figure 1), and there was no difference in the Δ of the Borg scale score for perception of dyspnea (p = 0.442). Neither Borg scale score presented a significant correlation with the 6MWD.
 Discussion
DiscussionIn the present study, the 6MWD was similar in COPD patients with and without LBM depletion, and no significant association was found between the indicators of body composition and the 6MWD. However, during the test, the patients with LBM depletion presented a significant increase in heart rate and in the sensation of lower limb fatigue in comparison with those without LBM depletion.
The influence of body composition on exercise tolerance varies according to the test used and the characteristics of the study. Some studies show that body mass composition is a determining factor of maximal exercise tolerance, as evaluated by the cycle ergometer test.(5,7,22) One group of authors studied 28 COPD patients submitted to maximal exercise testing (cycle ergometer), and observed that the patients with a body mass < 90% of the ideal presented a reduction in the aerobic capacity of skeletal muscle, and the authors considered this reduction to be a contributing factor for decreasing exercise tolerance.(22) Other studies also identified LBM-related attributes as a determining factor of VO2max in COPD patients.(5-7)
The results of the present study showed no significant influence of body mass composition on functional exercise tolerance, as evaluated based on the 6MWD. There have been few previous studies evaluating this association, and the results are inconclusive. A previous study showed that LBM, MIP and PaO2 explained 60% of the variation in the distance covered in 12 min,(8) and a recent study showed a significant positive correlation between the 6MWD and the LBMI (r2 = 0.42; p = 0.0001).(9)
However, two other studies showed no significant association between 6MWD and percentage of ideal weight or BMI.(23,24) In addition, some previous studies have shown that the increase in ventilatory demand, revealed by the increase in dyspnea, is more pronounced during walk tests than during cycle ergometer tests, and suggest that the walking capacity depends predominantly on airflow limitation.(25-27) In our study, airflow limitation, as evaluated based on FEV1 and on the sensation of dyspnea, was similar between the two groups (patients with and without LBM depletion) and might explain the similar 6MWD values. Although the patients with LBM depletion presented a significantly greater sensation of lower limb fatigue than did those without LBM depletion, the influence of this sensation on exercise tolerance could not be determined for the patients evaluated in the present study.
The correlation between the FEV1 (%) and 6MWD values found in the COPD patients evaluated in the present study was similar to that described in a previous study,(28) with values distributed along the identity line. Those authors also showed that 6MWD decline can occur regardless of worsening of FEV1, and suggest that the two outcomes evaluate different functional aspects of COPD patients. In fact, FEV1 evaluates the severity of the airway obstruction, whereas the 6MWD is influenced by functional limitation, symptoms, systemic manifestations of the disease and cardiopulmonary performance.
No significant correlation was found between LBM depletion and quality of life. There was no significant difference between the patients with LBM depletion and those without in terms of total SGRQ score or SGRQ domain scores, nor was there any significant difference in terms of AQ20 scores. These results are similar to those obtained by another group of authors,(29) who studied 273 COPD patients without LBM depletion and 102 COPD patients with LBM depletion. Those authors also found that there was no significant difference between the groups in terms of SGRQ scores. However, another study showed that COPD patients with a BMI > 21 kg/m2 and an LBMI ≤ 15 kg/m2 (for females) or ≤ 16 kg/m2 (for males) presented an increase in the SGRQ impact and activity domain scores in comparison with those with a BMI ≤ 21 kg/m2 and an LBMI > 15 kg/m2 (for females) or > 16 kg/m2 (for males), with no significant difference between the groups in terms of the symptom domain scores.(30) Nevertheless, when the patients with a BMI ≤ 21 kg/m2 and an LBMI > 15 kg/m2 (for females) or > 16 kg/m2 (for males) were compared with those with a BMI ≤ 21 kg/m2 and an LBMI ≤ 15 kg/m2 (for females) or ≤ 16 kg/m2 (for males), there was a significant difference only in the activity domain scores, with no changes in the impact or symptom domain scores. These results should be interpreted with caution, since the number of patients evaluated in each group was very small.(30) Therefore, the effect of LBM on the quality of life of COPD patients remains undefined.
The results of the present study do not allow us to rule out the influence of peripheral muscle mass on VO2max, since the performance of the patients during maximal exercise tests on a cycle ergometer was not evaluated. Although the sensation of lower limb fatigue was evaluated, objective tests to determine the presence of peripheral muscle fatigue were not performed. In addition, the cardiopulmonary evaluation did not include more specific functional tests, and, therefore, impairment without clinical repercussion could not be identified. In fact, the difference between initial and final heart rate (before and after the 6MWT) was significantly greater in the COPD patients with LBM depletion, although there was no difference between the groups in terms of Δ arterial pressure. Although previous studies have shown that COPD patients present increased heart rate during the performance of physical activity on a cycle ergometer, with no significant changes during walking, we found no data comparing patients with LBM depletion and those without.(26,27)
In conclusion, the results of the present study show that, although LBM is an important prognostic factor in the evolution of COPD patients, LBM depletion had no influence on the functional exercise tolerance or quality of life of the patients studied. However, the patients with LBM depletion presented more pronounced lower limb fatigue during the 6MWT, underscoring the importance of the evaluation and treatment of systemic manifestations in COPD patients, as well as that of management directed at relieving airway obstruction.
References 1. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-55.
2. Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173(12):1390-413.
3. Dourado VZ, Tanni SE, Vale SA, Faganello MM, Sanchez FF, Godoy I. Systemic manifestations in chronic obstructive pulmonary disease. J Bras Pneumol. 2006;32(2):161-71.
4. Eisner MD, Blanc PD, Sidney S, Yelin EH, Lathon PV, Katz PP, et al. Body composition and functional limitation in COPD. Respir Res. 2007;8:7.
5. Baarends EM, Schols AM, Mostert R, Wouters EF. Peak exercise response in relation to tissue depletion in patients with chronic obstructive pulmonary disease. Eur Respir J. 1997;10(12):2807-13.
6. Palange P, Forte S, Onorati P, Paravati V, Manfredi F, Serra P, et al. Effect of reduced body weight on muscle aerobic capacity in patients with COPD. Chest. 1998;114(1):12-8.
7. Yoshikawa M, Yoneda T, Kobayashi A, Fu A, Takenaka H, Narita N, et al. Body composition analysis by dual energy X-ray absorptiometry and exercise performance in underweight patients with COPD. Chest. 1999;115(2):371-5.
8. Schols AM, Mostert R, Soeters PB, Wouters EF. Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax. 1991;46(10):695-9.
9. Ischaki E, Papatheodorou G, Gaki E, Papa I, Koulouris N, Loukides S. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest. 2007;132(1):164-9.
10. Standardization of spirometry--1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis. 1987;136(5):1285-98.
11. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725-34.
12. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-7.
13. Borg G. Escala CR10 de Borg. In: Borg G, editors. Escalas de Borg para a dor e esforço percebido. São Paulo: Manole; 2000. p. 43-47.
14. Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34(11):2540-5.
15. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23(5):1226-43.
16. Kyle UG, Pichard C, Rochat T, Slosman DO, Fitting JW, Thiebaud D. New bioelectrical impedance formula for patients with respiratory insufficiency: comparison to dual-energy X-ray absorptiometry. Eur Respir J. 1998;12(4):960-6.
17. Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147(5):1151-6.
18. Sousa TC, Jardim JR, Jones P. Validação do Questionário do Hospital Saint George na Doença Respiratória (SGRQ) em pacientes portadores de doença pulmonar obstrutiva crônica no Brasil. J Pneumol. 2000;26(3):119-8.
19. Camelier A, Rosa F, Jones P, Jardim JR. Validação do questionário de vias aéreas 20 ("Airways questionnaire 20" - AQ20) em pacientes portadores de doença pulmonar obstrutiva crônica (DPOC) no Brasil. J Pneumol. 2003; 29(1):28-35.
20. Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751-8.
21. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580-6.
22. Palange P, Forte S, Felli A, Galassetti P, Serra P, Carlone S. Nutritional state and exercise tolerance in patients with COPD. Chest. 1995;107(5):1206-12.
23. Gray-Donald K, Gibbons L, Shapiro SH, Martin JG. Effect of nutritional status on exercise performance in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;140(6):1544-8.
24. Oga T, Nishimura K, Tsukino M, Hajiro T, Ikeda A, Mishima M. Relationship between different indices of exercise capacity and clinical measures in patients with chronic obstructive pulmonary disease. Heart Lung. 2002;31(5):374-81.
25. Palange P, Forte S, Onorati P, Manfredi F, Serra P, Carlone S. Ventilatory and metabolic adaptations to walking and cycling in patients with COPD. J Appl Physiol. 2000;88(5):1715-20.
26. Man WD, Soliman MG, Gearing J, Radford SG, Rafferty GF, Gray BJ, et al. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(5):562-7.
27. Pepin V, Saey D, Whittom F, LeBlanc P, Maltais F. Walking versus cycling: sensitivity to bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(12):1517-22.
28. Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J. 2004;23(1):28-33.
29. Vermeeren MA, Creutzberg EC, Schols AM, Postma DS, Pieters WR, Roldaan AC, et al. Prevalence of nutritional depletion in a large out-patient population of patients with COPD. Respir Med. 2006;100(8):1349-55.
30. Mostert R, Goris A, Weling-Scheepers C, Wouters EF, Schols AM. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2000;94(9):859-67.
About the authorsNilva Regina Gelamo Pelegrino
Pulmonologist. State Hospital at Bauru, Bauru, Brazil.
Paulo Adolfo Lucheta
Physical Therapist. Pulmonary Rehabilitation Program, Botucatu School of Medicine, São Paulo State University, Botucatu, Brazil.
Renata Ferrari
Physical Therapist. Pulmonary Rehabilitation Program, Botucatu School of Medicine, São Paulo State University, Botucatu, Brazil.
Fernanda Figueirôa Sanchez
Physical Therapist. Department of Respiratory Therapy, Auxiliary Salesian Catholic University Center, Araçatuba, Brazil.
Márcia Maria Faganello
Physical Therapist. Department of Respiratory Therapy, Unisalesiano de Lins, Lins, Brazil.
Irma de Godoy
Full Professor of Pulmonology. Department of Clinical Medicine, Botucatu School of Medicine, São Paulo State University, Botucatu, Brazil.
Study carried out at the Botucatu School of Medicine, São Paulo State University, Botucatu, Brazil.
Correspondence to: Nilva Regina Gelamo Pelegrino. Rua Sergipe, 1-29, apto. 24B, Vila Cárdia, CEP 17013-670, Bauru, SP, Brasil.
Tel 55 14 3103-7777. E-mail: nilvap@itelefonica.com.br
Financial support: None.
Submitted: 13 March 2008. Accepted, after review: 12 June 2008.






