ABSTRACT
Abstract
Lemierre's syndrome is characterized by acute oropharyngeal infection, complicated by internal jugular venous thrombosis secondary to septic thrombophlebitis, and by metastatic infections in various distant organs-most commonly in the lungs. We report a case of Lemierre's syndrome in a 56-year-old female who presented with right-sided neck mass and fever. Right internal jugular venous thrombosis was demonstrated on an ultrasound. A computed tomography scan of the chest revealed multiple opacities throughout both lungs. An open surgical biopsy was performed due to suspicion of pulmonary metastases. Anatomopathological examination revealed septic emboli in lung parenchyma. Retrospectively, the patient reported a history of pharyngitis two weeks prior to hospitalization. After the diagnosis had been made, the patient was treated with broad-spectrum antibiotics (cefuroxime for 7 days and azithromycin for 5 days; subsequently, because fever persisted, cefepime for 7 days). One month later, a computed tomography scan of the chest revealed resolution of the opacities.
Keywords:
Pulmonary embolism; Lung abscess; Thrombophlebitis; Jugular veins; Pharyngitis.
RESUMO
A síndrome de Lemierre é caracterizada pela infecção aguda da orofaringe, complicada por trombose venosa jugular interna secundária à tromboflebite séptica, e por infecções metastáticas a vários órgãos distantes-mais freqüentemente os pulmões. Relatamos um caso de síndrome de Lemierre em uma mulher de 56 anos que se apresentou com massa cervical à direita e febre. Trombose venosa jugular interna foi demonstrada na ecografia. A tomografia computadorizada de tórax revelou múltiplas opacidades em ambos os pulmões. Uma biópsia pulmonar cirúrgica foi realizada por suspeita de metástases pulmonares. O exame anatomopatológico revelou êmbolos sépticos em parênquima pulmonar. Retrospectivamente, a paciente relatou história de faringite duas semanas antes da hospitalização. Após o diagnóstico, foi tratada com antibióticos de amplo espectro (cefuroxima por 7 dias e azitromicina por 5 dias e, posteriormente, devido à persistência de febre, cefepime por 7 dias). A tomografia computadorizada de tórax, realizada um mês após, mostrou resolução das opacidades.
Palavras-chave:
Embolia pulmonar; Abscesso pulmonar; Tromboflebite; Veias jugulares; Faringite.
IntroductionLemierre's syndrome (necrobacillosis or postanginal sepsis) is caused by acute oropharyngeal infection with secondary internal jugular vein (IJV) septic thrombophlebitis, often complicated by metastatic infections. It is a severe disease caused by the anaerobic bacterium Fusobacterium necrophorum. It typically occurs in healthy adolescents and young adults.
In most cases, the infection originates in the palatine tonsils and circumjacent tissues. However, it can also initiate as pharyngitis, parotitis, otitis media, sinusitis, odontogenic infection or mastoiditis. Subsequently, there is infection of the lateral pharyngeal space, through which the IJV passes, leading to IJV septic thrombophlebitis. The bacteremia is complicated by septic embolus migration to various sites, such as the lungs (most commonly), joints and bones. In the pre-antibiotic era, this syndrome was not uncommon, and had a fulminant evolution, typically resulting in death within 7 to 15 days. Although Lemierre's syndrome is currently rare, there is evidence of its resurgence in recent years, and there are a number of case reports in the literature. This is likely related to the fact that the use of antimicrobial agents for the treatment of upper respiratory tract infections is restricted in order to avoid the unnecessary use of such agents in cases of viral etiology. No cases similar to the one reported here have been described in Brazil.(1-4)
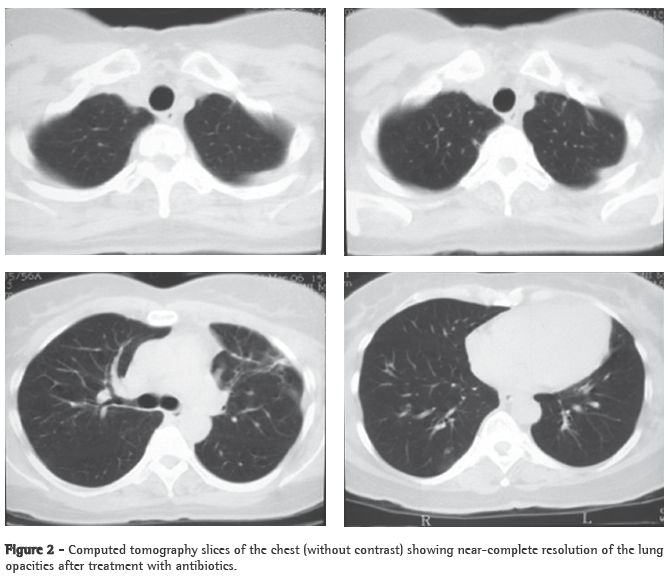
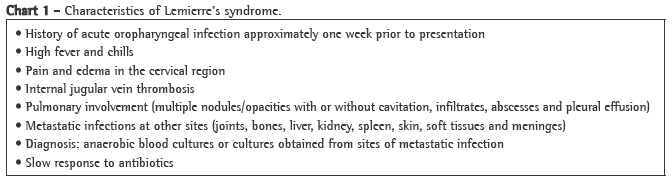
Case reportA 56-year-old Caucasian female sought treatment in the emergency room presenting a painful, right-sided neck tumor for 5 days. The patient reported a dry cough and high fever for 3 days. She described herself as a nonsmoker. She was hypertensive and diabetic. She had experienced a myocardial infarction 3 years prior. At admission, the patient was dehydrated and afebrile (axillary temperature, 36.7°C). She presented normal respiration. Laboratory tests revealed increased erythrocyte sedimentation rate (92 mm). Ultrasound of the neck showed IJV thrombosis, and anticoagulation was started on post-admission day 4. A chest X-ray demonstrated at least two nodules in the left lung, one apparently cavitated and one with a diameter of 18 mm at its base, as well as an irregular lesion, 25 mm in diameter, in the right upper lung lobe. A transesophageal echocardiogram, performed in order to rule out endocarditis, demonstrated no vegetation. Due to the hypothesis of pneumonia, empirical treatment with oral cefuroxime (500 mg every 12 h) was started. A computed tomography scan of the chest revealed multiple bilateral lung opacities, with an aspect suggestive of metastatic implants (Figure 1). The patient was then submitted to fiberoptic bronchoscopy, the findings of which were normal. In the microbiological analysis of the bronchoalveolar lavage fluid, testing for acid-fast bacilli and fungi were negative, as were the cytopathologic study and cultures. Since the working diagnosis was metastatic neoplasm, the patient underwent surgical lung biopsy. The anatomopathological examination of the sample showed chronic suppurative inflammation with organizing abscesses in lung parenchyma. In view of the hypothesis of Lemierre's syndrome caused by jugular thrombosis accompanied by septic pulmonary embolism, the patient was questioned regarding the occurrence of tonsillitis at the onset of the disease. The patient had no recollection, but her daughter remembered that the patient had used oral amoxicillin (500 mg every 8 h for 10 days) for the treatment of tonsillitis some days prior to hospitalization. At hospital admission, the patient received a course of intravenous cefuroxime (750 mg every 8 h for 7 days) concomitantly with oral azithromycin (500 mg once a day for 5 days). Subsequently, because fever persisted, she was treated with cefepime (1,000 mg every 12 h for 7 days). A control computed tomography scan of the chest performed after this course of antibiotics showed nearly complete resolution of the opacities (Figure 2). The patient had been afebrile since the third day of the new course of antibiotics. No microorganisms were isolated from blood cultures or bronchoalveolar lavage cultures. Since there is no evidence that it is beneficial in cases of Lemierre's syndrome, anticoagulation was discontinued after 20 days.

 Discussion
DiscussionLemierre's syndrome seems to have been relatively common in the pre-antibiotic era. Although it remains a rare disease (incidence of 1 case/1,000,000 individuals/year), there has been an increase in the number of reported cases since 1990. One hypothesis for the resurgence of the disease is the reduction in the prescription of antibiotics for tonsillitis. The increase in the number of cases of bacteremia caused by Fusobacterium sp. can also be due to improvements in anaerobic blood culture techniques.(1)
Fusobacteria are anaerobic gram-negative bacteria that are always present in the normal flora of the upper respiratory tract, of the female genital tract and of the gastrointestinal tract. Due to the necrotic abscesses produced by Fusobacterium necrophorum, the condition became known as necrobacillosis. The first case was described in 1900 by Courmont & Cade. The condition was better characterized in 1936 by André Lemierre, who, in a lecture on anaerobic septicemia, divided patients into six groups according to the origin of the infection: inflammatory lesions of the nasopharynx (tonsillar/peritonsillar abscess); lesions of the mouth and mandible; otitis media or mastoiditis; purulent postpartum endometritis; appendicitis; and urinary tract infection. The patients in the first group were described as having "postanginal anaerobic septicemia". Those patients were young and previously healthy, as well as having pharyngotonsillitis or peritonsillar abscess, often followed by edema and increased sternocleidomastoid muscle sensitivity due to IJV septic thrombophlebitis.
As can be seen in Chart 1, high fever and chills develop within one week; subsequently, there are metastatic abscesses in the lung, bones, joints, skin and soft tissues.(1) Our patient was not in the age bracket classically affected by this syndrome and was not previously healthy (she had arterial hypertension and diabetes mellitus). However, in the literature, there have been some reports of older patients with comorbidities who had this syndrome.(1,5,6)
The origin of the infection might not be the throat but rather the ear, the mastoid, or even a dental infection. Some authors do not require isolation of Fusobacterium sp. for the diagnosis.(1)
Considering that Fusobacterium sp. is part of the normal flora, there should be a precipitating factor of invasive infection, such as mucosal damage caused by viral or bacterial pharyngitis. Recent infection with Epstein-Barr virus can induce immunosuppression, facilitating secondary bacterial infection.(1) One case of Lemierre's syndrome in a patient with variations in coagulation genes associated with thrombotic events has been reported in the literature, suggesting a genetic predisposition for the development of this syndrome.(7) Cases associated with coagulopathy and factor XII deficiency have also been described.(8,9)
It is difficult to identify Fusobacterium sp., which can be confused with Bacteroides sp. It is often found in association with other pathogens (33% of the cases). Other organisms occasionally found are Streptococcus sp., Bacteroides sp., Peptostreptococcus sp. and Eikenella corrodens(1,2)
Klebsiella pneumoniae has been described in a diabetic patient.(8) In the case reported here, no microorganisms were identified. This can be due to the previous use of antibiotics, as well as to the fact that anaerobic blood cultures were not requested, since Lemierre's syndrome was not considered among the initial working diagnoses.
At the onset of the disease, the symptoms are fever (39-41°C) and chills, which occur 4 to 5 (up to 12) days after the onset of pharyngitis. Pain and stiffness can also occur. Unilateral or bilateral cervical lymphadenopathy can also be present, usually in the anterior triangle. Edema and pain in the mandible angle or located anterior and parallel to the sternocleidomastoid muscle reflect the development of IJV thrombophlebitis, which occurs in 26% to 45% of the cases. The patient in the present case began to present symptoms 5 days after the onset of pharyngitis.(1)
Pulmonary involvement in this syndrome is extremely common (seen in up to 97% of the cases). Pulmonary lesions can manifest on the first day of sepsis. There can be intense pleuritic pain with dyspnea, and hemoptysis often occurs. Pulmonary auscultation can reveal localized crackling rales and pleural friction rub. Chest X-rays typically show multiple bilateral opacities and small pleural effusions. It is possible to detect cavitation on the first X-ray. There can be rapid progression of the lesions, even with the use of antibiotics. Empyema develops in 10% to 15% of cases. Abscess, pneumothorax and pneumatoceles have been described. After contrast administration, there is peripheral enhancement of the lesions with central areas of decreased attenuation. The differential diagnosis should be made with pneumonia (acute bacterial, atypical, aspiration and staphylococcal pneumonia).(1,2,11-14)
Patients present leukocytosis with neutrophilia. Liver function test results are altered in 50% of the cases. It takes Fusobacterium sp. at least 48 h, and sometimes up to 7 days, to grow in blood cultures, and
Fusobacterium sp. is also identified by molecular biology.(1) Ultrasound or tomography scans are used in order to confirm IJV thrombophlebitis.(1,12)
The response to antibiotics is slow. The mean time between the initiation of treatment and the resolution of fever ranges from 8 to 12 days.
Fusobacterium sp. is generally sensitive to penicillin, clindamycin, metronidazole, cephalosporins, tetracycline and chloramphenicol. The initial regimen suggested is penicillin and metronidazole for at least two weeks. In cases of penicillin treatment failure, the options are ticarcillin/clavulanate and imipenem In the case reported here, amoxicillin, cefuroxime, cefepime and azithromycin were used. The patient became afebrile only after the third antibiotic regimen, which might be due to the short duration of antibiotic use, rather than to antibiotic treatment failure.
The use of anticoagulants is controversial. It is reserved for patients with retrograde progression to the cavernous sinus. Only in cases of persistent septic embolization is IJV ligation indicated, even if antibiotic therapy has been initiated.(1)
In the pre-antibiotic era, the prognosis of this syndrome was poor. With antibiotic treatment, despite the severity of sepsis, complete recovery is common, and mortality rates range from 0% to 18%.(1)
The presence of pulmonary opacities in a patient with fever and concomitant jugular venous thrombosis should raise the suspicion of Lemierre's syndrome.
References 1. Riordan T, Wilson M. Lemierre's syndrome: more than a historical curiosa. Postgrad Med J. 2004;80(944):328-34.
2. Golpe R, Marín B, Alonso M. Lemierre's syndrome (necrobacillosis). Postgrad Med J. 1999;75(881):141-4.
3. Bliss SJ, Flanders SA, Saint S. Clinical problem-solving. A pain in the neck. N Engl J Med. 2004;350(10):1037-42.
4. Riordan T. Human infection with Fusobacterium necrophorum (Necrobacillosis), with a focus on Lemierre's syndrome. Clin Microbiol Rev. 2007;20(4):622-59.
5. Shibasaki Warabi Y, Yoshikawa H, Idezuka J, Yamazaki M, Onishi Y. Cerebral infarctions and brain abscess due to Lemierre syndrome. Intern Med. 2005;44(6):653-6.
6. Morizono S, Enjoji M, Sonoda N, Fukushima M, Kuniyoshi M, Kotoh K, et al. Lemierre's syndrome: Porphyromonas asaccharolytica as a putative pathogen. Intern Med. 2005;44(4):350-3.
7. Singaporewalla RM, Clarke MJ, Krishnan PU, Tan DE. Is this a variant of Lemierre's syndrome? Singapore Med J. 2006;47(12):1092-5.
8. Constantin JM, Mira JP, Guerin R, Cayot-Constantin S, Lesens O, Gourdon F, et al. Lemierre's syndrome and genetic polymorphisms: a case report. BMC Infect Dis. 2006;6:115.
9. Georgopoulos S, Korres S, Riga M, Balatsouras D, Kotsis G, Ferekidis E. Lemierre's syndrome associated with consumption coagulopathy and acute renal failure: a case report. J Laryngol Otol. 2008;122(5):527-30.
10. Hlibczuk V. Lemierre's syndrome complicating bacterial pharyngitis in a patient with undiagnosed factor XII deficiency [abstract]. J Emerg Med. 2007;32(4):365-9.
11. Screaton NJ, Ravenel JG, Lehner PJ, Heitzman ER, Flower CD. Lemierre syndrome: forgotten but not extinct--report of four cases. Radiology. 1999;213(2):369-74.
12. Cook RJ, Ashton RW, Aughenbaugh GL, Ryu JH. Septic pulmonary embolism: presenting features and clinical course of 14 patients. Chest. 2005;128(1):162-6.
13. Nguyen-Dinh KV, Marsot-Dupuch K, Portier F, Lamblin B, Lasjaunias P. Lemierre syndrome: usefulness of CT in detection of extensive occult thrombophlebitis. J Neuroradiol. 2002;29(2):132-5.
14. UpToDate [homepage on the Internet]. Massachusetts: UpToDate, Inc. c2008. [updated 2007 Jan 3; cited 2008 Feb 28]. Available from: http://www.uptodateonline.com
* Study carried out at the Porto Alegre Hospital de Clínicas, Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - Porto Alegre, Brazil.
1. Pulmonologist. Porto Alegre Hospital de Clínicas, Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - Porto Alegre, Brazil.
2. Attending Physician. Department of Pulmonology, Porto Alegre Hospital de Clínicas, Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - Porto Alegre, Brazil.
3. Associate Professor. Department of Internal Medicine, Porto Alegre Hospital de Clínicas, Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - Porto Alegre, Brazil.
4. Resident in Internal Medicine. Porto Alegre Hospital de Clínicas, Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - Porto Alegre, Brazil.
Correspondence to: Marcelo Basso Gazzana. Serviço de Pneumologia, Hospital de Clínicas de Porto Alegre, Rua Ramiro Barcelos, 2350, 2º andar, Sala 2050, CEP 90035-003, Porto Alegre, RS, Brasil.
Tel 55 51 2101-8241. E-mail: marcgaz@terra.com.br
Financial support: None.
Submitted: 5 March 2008. Accepted, after review: 17 April 2008.






