Rafaella Rezende Rondelli, Simone Dal Corso, Alexandre Simões, Carla Malaguti
ABSTRACT
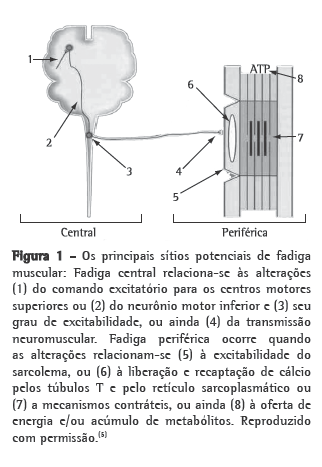
It has been well established that, in addition to the pulmonary involvement, COPD has systemic consequences that can lead to peripheral muscle dysfunction, with greater muscle fatigue, lower exercise tolerance and lower survival in these patients. In view of the negative repercussions of early muscle fatigue in COPD, the objective of this review was to discuss the principal findings in the literature on the metabolic and bioenergy determinants of muscle fatigue, its functional repercussions, as well as the methods for its identification and quantification. The anatomical and functional substrate of higher muscle fatigue in COPD appears to include lower levels of high-energy phosphates, lower mitochondrial density, early lactacidemia, higher serum ammonia and reduced muscle perfusion. These alterations can be revealed by contraction failure, decreased firing rates of motor units and increased recruitment of motor units in a given activity, which can be functionally detected by a reduction in muscle strength, power and endurance. This review article also shows that various types of muscle contraction regimens and protocols have been used in order to detect muscle fatigue in this population. With this understanding, rehabilitation strategies can be developed in order to improve the resistance to muscle fatigue in this population.
Keywords: Pulmonary disease, chronic obstructive; Neuromuscular manifestations; Muscle fatigue; Exercise tolerance; Energy metabolism; Evaluation.
RESUMO
Está bem estabelecido que, além do acometimento pulmonar, a DPOC apresenta consequências sistêmicas que podem convergir para a disfunção muscular periférica, com maior fadigabilidade muscular, menor tolerância ao exercício e menor sobrevida nesses pacientes. Tendo em vista as repercussões negativas da fadiga muscular precoce na DPOC, esta revisão teve como objetivo discutir os principais achados da literatura relacionados aos seus determinantes metabólicos e bioenergéticos e suas repercussões funcionais, bem como seus métodos de identificação e de quantificação. O substrato anatomofuncional da maior fadigabilidade muscular na DPOC parece incluir a redução dos níveis de fosfatos de alta energia, a redução da densidade mitocondrial, a lactacidemia precoce, o aumento da amônia sérica e a perfusão muscular reduzida. Essas alterações podem ser evidenciadas por falência de contração, redução da taxa de disparo das unidades motoras e maior recrutamento de unidades motoras numa dada atividade, exteriorizando-se funcionalmente por redução da força, potência e resistência musculares. Esta revisão mostra também que diversos tipos de regimes contráteis e protocolos têm sido utilizados com o intuito de detectar fadiga nessa população. A partir de tais conhecimentos, estratégias reabilitadoras podem ser traçadas visando o aumento da resistência à fadiga muscular nessa população.
Palavras-chave: Doença pulmonar obstrutiva crônica; Manifestações neuromusculares; Fadiga muscular; Tolerância ao exercício; Metabolismo energético; Avaliação.
Introdução