Priscila Kessar Cordoni, Danilo Cortozi Berton, Selma Denis Squassoni, Maria Enedina Aquino Scuarcialupi, José Alberto Neder, Elie Fiss
ABSTRACT
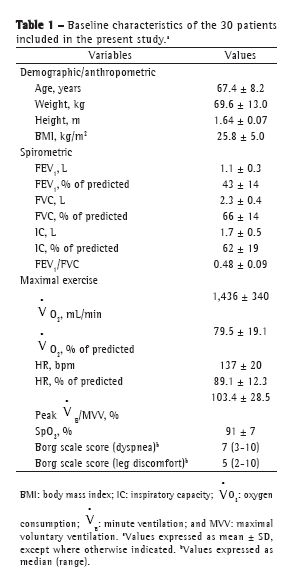
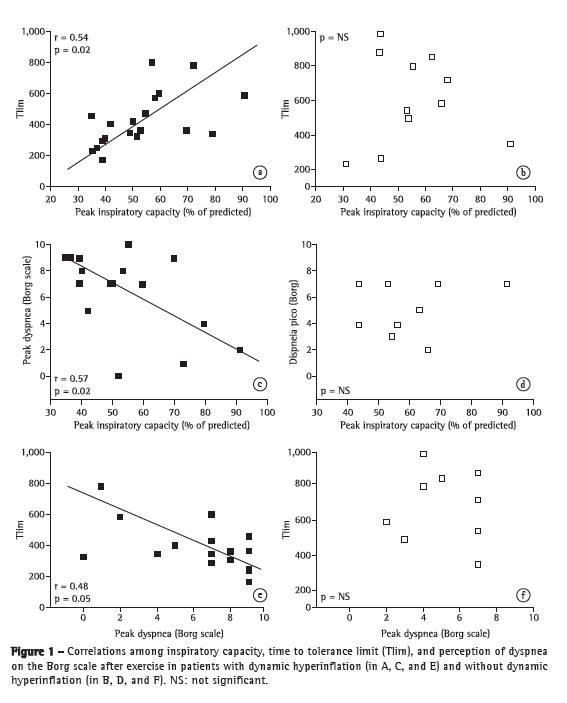
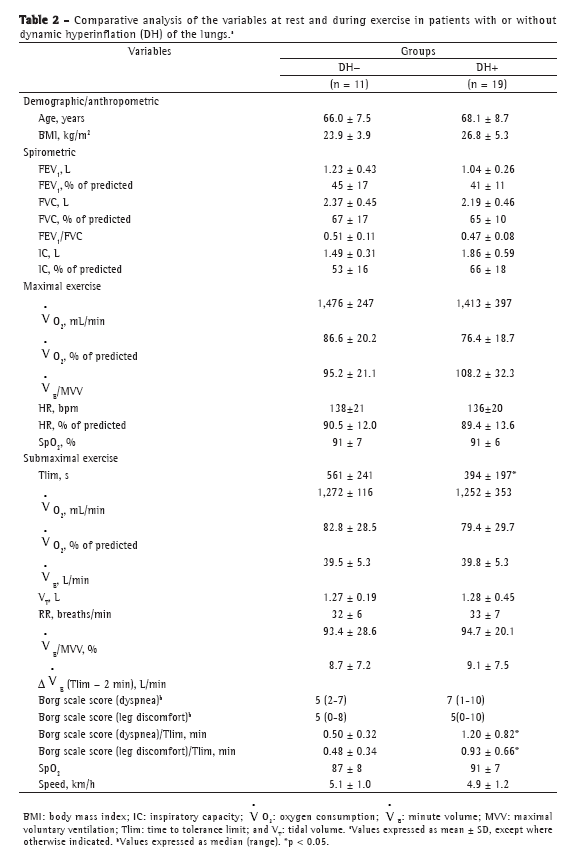
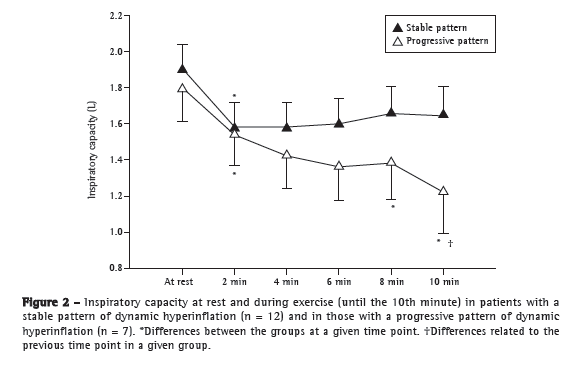
Objective: To characterize the presence, extent, and patterns of dynamic hyperinflation (DH) during treadmill exercise testing in patients with moderate to severe COPD. Methods: This was a cross-sectional study involving 30 non-hypoxemic patients (FEV1= 43 ± 14% of predicted) who were submitted to a cardiopulmonary exercise test on a treadmill at a constant speed (70-80% of maximum speed) to the tolerance limit (Tlim). Serial inspiratory capacity (IC) maneuvers were used in order to assess DH. Results: Of the 30 patients studied, 19 (63.3%) presented with DH (DH+ group), having greater pulmonary function impairment at rest than did those without DH (DH− group). None of the variables studied correlated with exercise tolerance in the DH− group, whereas Tlim, IC, and perception of dyspnea during exercise did so correlate in the DH+ group (p < 0.05). In the DH+ group, 7 and 12 patients, respectively, presented with a progressive and a stable pattern of DH (ΔICTlim,2min = −0.28 ± 0.11 L vs. 0.04 ± 0.10 L; p < 0.01). Patients with a progressive pattern of DH presented with higher perception of dyspnea/Tlim rate and lower exercise tolerance than did those with a stable pattern (354 ± 118 s and 465 ± 178 s, respectively; p < 0.05). Conclusions: The presence of DH is not a universal phenomenon during walking in COPD patients, even in those with moderate to severe airflow limitation. In the patients who presented DH, a progressive pattern of DH had a greater impact on exercise tolerance than did a stable pattern of DH.
Keywords: Pulmonary disease, chronic obstructive; Exercise; Exercise test; Inspiratory capacity.
RESUMO
Objetivo: Caracterizar a presença, extensão e padrões de hiperinsuflação dinâmica (HD) durante teste em esteira rolante em pacientes com DPOC moderada a grave. Métodos: Estudo transversal com 30 pacientes não hipoxêmicos (VEF1= 43 ± 14% do previsto) submetidos a teste cardiopulmonar de exercício em esteira rolante em velocidade constante (70-80% da velocidade máxima) até o limite da tolerância (Tlim). Manobras seriadas de capacidade inspiratória (CI) foram utilizadas para avaliação da HD. Resultados: Dos 30 pacientes estudados, 19 (63,3%) apresentaram HD (grupo HD+), que apresentaram maior comprometimento funcional em repouso do que os pacientes sem HD (grupo HD−). Nenhuma das variáveis obtidas relacionou-se com a tolerância ao exercício no grupo HD−, enquanto Tlim, CI e percepção de dispneia ao esforço foram significativamente correlacionados no grupo HD+ (p < 0,05). No grupo HD+, 7 e 12 pacientes, respectivamente, apresentaram padrão progressivo e estável de HD (ΔCITlim,2min = −0,28 ± 0,11 L e 0,04 ± 0,10 L; p < 0,01). Pacientes com padrão progressivo de HD apresentaram maior relação percepção de dispneia/Tlim e menor tolerância ao exercício do que aqueles com padrão estável (354 ± 118 s e 465 ± 178 s, respectivamente; p < 0,05). Conclusões: A HD não é um fenômeno universal durante a caminhada em pacientes com DPOC, mesmo que apresentem obstrução ao fluxo aéreo de graus moderado a acentuado. Nos pacientes que apresentaram HD, um padrão progressivo de HD teve maior repercussão na tolerância ao exercício do que um padrão estável de HD.
Palavras-chave: Doença pulmonar obstrutiva crônica; Exercício; Teste de esforço; Capacidade inspiratória.
Introduction