TO THE EDITOR: Thymic epithelial tumors with pleural dissemination (Masaoka stage IVa thymomas) or malignant pleural implants are associated with unfavorable prognosis. Despite advances in systemic therapy, open cytoreduction surgery (CRS) remains pivotal for durable disease control in selected patients. However, negative microscopic margins are rarely achieved, contributing to high recurrence rates.
Hyperthermic intrathoracic chemotherapy (HITHOC) has emerged as a promising intraoperative strategy to enhance locoregional control. HITHOC delivers high concentrations of cytotoxic agents—most commonly cisplatin (150-225 mg/m2)—via a heated perfusate (42°C) maintained for 60 min within a closed pleural circuit, thus ensuring improved tissue penetration and minimal systemic absorption.(1-3)
Although most experiences with HITHOC involve open approaches, the combined use of robot-assisted thoracic surgery and HITHOC remains underreported. Robotic platforms provide enhanced visualization, superior instrument articulation, and improved access to pleural recesses. Here, we describe a standardized, step-by-step protocol for robotic CRS followed by HITHOC, implemented at a high-volume thoracic oncology center in Latin America.
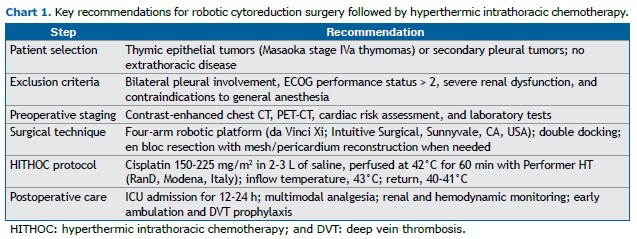
Eligible patients include those with resectable pleural metastases from thymic epithelial tumors or select secondary pleural malignancies without extrathoracic spread. Exclusion criteria include bilateral pleural involvement, an ECOG performance status > 2, significant renal dysfunction, and contraindications to general anesthesia. Preoperative staging includes contrast-enhanced chest CT, PET-CT, pulmonary function tests (including DLCO measurement), cardiac evaluation in accordance with the American College of Cardiology/American Heart Association guidelines, and laboratory assessment.
A thoracic epidural catheter is placed prior to induction. General anesthesia is induced with target-controlled infusions of propofol and remifentanil, as well as rocuronium for neuromuscular blockade. A double-lumen endotracheal tube is inserted for single-lung ventilation. Arterial and central venous access are secured as indicated. Prophylactic cefuroxime (1.5 g i.v.) is administered before incision, followed by 750 mg every 4 h intraoperatively and every 8 h postoperatively.
Protective ventilation strategies include low tidal volumes (4-6 mL/kg), a PEEP of 5-10 cmH2O, and FiO2 titration to maintain an SpO2 > 92%. Lung isolation is confirmed via bronchoscopy. CPAP may be applied to the nonventilated lung if needed. Intraoperative monitoring includes serial evaluation of hemoglobin, electrolytes, glucose, lactate, and hemodynamic parameters, particularly during perfusion, because of expected mediastinal fluid shifts.
A four-arm robotic platform (da Vinci Xi; Intuitive Surgical, Sunnyvale, CA, USA) is employed, with port placement adapted from robotic lobectomy to maximize pleural exposure. In cases of extensive disease, a double-docking strategy is utilized to facilitate full parietal pleurectomy. En bloc resection of diaphragmatic or pericardial surfaces is performed as needed, followed by reconstruction with nonabsorbable mesh or bovine pericardium.
At the conclusion of CRS, two chest tubes are positioned: the inflow drain is placed at the diaphragmatic base, parallel to the mediastinum, whereas the outflow drain is positioned at the thoracic apex, along the parietal pleura. This configuration facilitates a unidirectional upward flow of the heated chemotherapeutic perfusate, optimizing its distribution and contact time with pleural surfaces. Continuous circulation ensures thermal homogeneity and prevents fluid stagnation, thus reducing the risk of locoregional recurrence. The thoracic cavity is then hermetically sealed to prevent chemotherapeutic leakage and minimize the risk of skin or wound contamination.
Approximately 30 min before completing CRS, the surgeon communicates with the perfusionist to initiate the setup of a dedicated closed-circuit hyperthermic system (Performer HT; RanD, Modena, Italy). Preparation includes installation of a sterile disposable kit (including inflow/outflow catheters, temperature sensors, and connectors); priming with isotonic saline; and gradual heating of the circuit to 42°C. Once connected to the patient, the pleural cavity is filled via the inflow chest tube. Air clearance and thermal equilibrium are ensured before initiation of chemoperfusion. The target inflow temperature is 43°C, and the target return temperature is 40-41°C (mean, 41.5°C). Cisplatin is administered within a sealed system to avoid environmental exposure. After 60 min of continuous perfusion, the circuit is flushed and the intrapleural solution drained via the outflow chest tube (Figure 1).
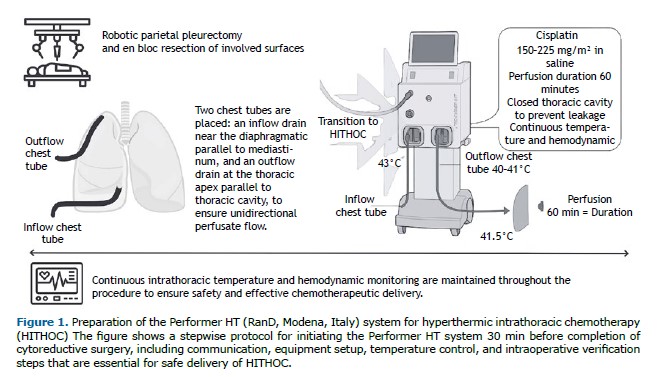
Throughout perfusion, intrathoracic temperature, flow rate, and core body temperature are continuously monitored to ensure procedural safety and protocol adherence.
All patients are monitored in the ICU for 12-24 h. Postoperative care includes multimodal analgesia via epidural or patient-controlled analgesia, renal function monitoring, and hemodynamic monitoring. Thromboprophylaxis, incentive spirometry, and early ambulation are initiated on postoperative day one. Surveillance imaging is performed in accordance with the institutional protocol (Chart 1).
This article presents the first standardized description of robotic CRS followed by HITHOC in Latin America. The integration of robotic technology into the HITHOC workflow enhances the precision of pleural dissection while potentially reducing morbidity. Given the paucity of published data on minimally invasive approaches for pleural malignancies, the reproducibility and safety of this technique should be further validated through prospective studies. Emphasis should be placed on protocol standardization, rigorous patient selection, and long-term outcome analysis—including survival and quality of life endpoints—to support broader adoption.
ETHICS STATEMENTThis study was approved by the local institutional review board (Protocol no. 82427124500000071). The requirement for written informed consent was waived because there were no participants.
FINANCIAL SUPPORTNone.
AUTHOR CONTRIBUTIONSPDD’A and GV: conceptualization; data curation; formal analysis; methodology; and writing. RMT: conceptualization; methodology; supervision; and writing—review and editing. BMB: writing—original draft; and writing—review and editing.
CONFLICTS OF INTERESTNone declared.
REFERENCES 1. Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1999;117(1):54-65. https://doi.org/10.1016/S0022-5223(99)70469-1
2. Ambrogi MC, Bertoglio P, Aprile V, Pompeo E, Mineo TC. Diaphragm and lung-preserving surgery with hyperthermic chemotherapy for malignant pleural mesothelioma: a 10-year experience. J Thorac Cardiovasc Surg. 2018;155(4):1857-66. https://doi.org/10.1016/j.jtcvs.2017.10.070
3. Ried M, Eichhorn M, Winter H, Grützner U, Lindner M, Hatz RA, et al. Expert recommendation for the implementation of hyperthermic intrathoracic chemotherapy (HITHOC) in Germany [Article in German]. Zentralbl Chir. 2020;145(1):89-98. https://doi.org/10.1055/a-0934-7806
4. Migliore M, Nardini M. Does cytoreduction surgery and hyperthermic intrathoracic chemotherapy prolong survival in patients with N0-N1 non-small cell lung cancer and malignant pleural effusion? Eur Respir Rev. 2019;28(153):190018. https://doi.org/10.1183/16000617.0018-2019
5. Romano G, Zirafa CC, Ceccarelli I, Elia G, Davini F, Melfi F. Update in the treatment of pleural tumors: robotic surgery combined with hyperthermic intrathoracic chemother-apy. Cancers (Basel). 2024;16(9):1691. https://doi.org/10.3390/cancers16091691




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Pocket
Pocket