ABSTRACT
Objective: To evaluate, using pulmonary function tests, the effectiveness of formoterol as a bronchodilator at 30 min after its administration
in patients with poorly reversible COPD. Methods: A prospective study including 40 COPD patients not responding to the short-acting
bronchodilator used in the spirometric test-variation of less than 200 mL and less than 7% of predicted in forced expiratory volume in
one second (FEV1). All patients were classified as having stage II, III, or IV COPD (Brazilian Thoracic Society/Global Initiative for Chronic
Obstructive Lung Disease) and presented FEV1 ≤ 70% of predicted value. The patients were randomized into two groups of 20, with similar
clinical characteristics, receiving, via a dry powder inhaler, either formoterol or a placebo. The pulmonary function testing (plethysmography)
was repeated at 30 min after formoterol or placebo administration. Results: In the formoterol group, the mean values obtained for FEV1,
inspiratory capacity, and forced vital capacity were significantly greater than those obtained in the placebo group (p = 0.00065, p = 0.05,
and p = 0.017, respectively), whereas that obtained for airway resistance was significantly lower (p = 0.010). Less pronounced differences
were observed for residual volume, vital capacity and specific airway conductance, which were lower, higher and higher, respectively, in the
formoterol group. Conclusions: In COPD patients not responding to the short-acting bronchodilator used in the spirometric test, formoterol
promoted significant improvement in lung function at 30 min after of administration. Further studies are required to confirm whether
formoterol can also be used as a medication for immediate relief of symptoms in COPD.
Keywords:
Chronic obstructive pulmonary disease; Respiratory function tests; Bronchodilator agents.
RESUMO
Objetivo: Avaliar, por meio de provas de função pulmonar, a eficácia broncodilatadora do formoterol após 30 min de sua administração em
portadores de doença pulmonar obstrutiva crônica (DPOC) com pouca reversibilidade. Métodos: Estudo prospectivo incluindo 40 pacientes
portadores de DPOC com resposta negativa ao broncodilatador de curta duração utilizado no teste espirométrico-variação menor que
200 mL e 7% do previsto do volume expiratório forçado no primeiro segundo (VEF1). Os pacientes encontravam-se nos estágios II, III ou
IV da DPOC (Sociedade Brasileira de Pneumologia e Tisiologia/Global Initiative for Chronic Obstructive Lung Disease) e apresentavam VEF1
≤ 70% do previsto. Foram randomizados em dois grupos de 20, com características clínicas semelhantes, e cada grupo recebeu formoterol ou
placebo por meio de inalador de pó seco. As provas de função pulmonar (por pletismografia) foram repetidas após 30 min da administração
de formoterol ou placebo. Resultados: Observaram-se aumento significativo de VEF1 (p = 0,00065), capacidade inspiratória (p = 0,05) e
capacidade vital forçada (p = 0,017) e redução significativa da resistência das vias aéreas (p = 0,010) no grupo formoterol, em comparação
ao grupo placebo, assim como menor redução do volume residual e menor aumento da capacidade vital e da condutância específica das vias
aéreas. Conclusões: Em portadores de DPOC com resposta negativa ao broncodilatador de curta duração utilizado no teste espirométrico, o
formoterol levou a uma melhora significativa da função pulmonar após 30 min de sua administração. Estudos posteriores serão necessários
para determinar se esse fármaco pode ser utilizado também como medicação de alívio imediato dos sintomas em DPOC.
Palavras-chave:
Doença pulmonar obstrutiva crônica; Testes de função respiratória, Broncodilatadores.
IntroductionChronic obstructive pulmonary disease (COPD) is characterized by progressive airflow obstruction that is not entirely reversible.(1,2) Current COPD guidelines recommend the prescription of bronchodilators as an important pharmacological measure in the treatment of this condition.(1,2)
Formoterol is a potent, long-acting (12-h) β2-agonist with a rapid onset of action, which has been verified in a number of studies involving asthma patients. In some instances, however, when the drug was used in patients with COPD, short-term and long-term functional benefits have been reported.(3) The onset of action of the formoterol bronchodilator effect in COPD can be as immediate as that observed for short-acting β2-agonists such as salbutamol.(4)
In clinical practice, patients report a subjective improvement of symptoms after the use of formoterol,(5) although such improvements are not always confirmed through the bronchodilator reversibility testing commonly in use.(6) This failure to demonstrate an effect, as defined by measuring forced expiratory volume in one second (FEV1), might result from an early collapse of the airways, which can lead to the underestimation of the bronchodilator effect in the more peripheral airways, where resistance is more marked in COPDs.(7) Recent studies focusing on variables other than FEV1 to investigate the immediate effect of formoterol as a bronchodilator in COPD, including volume analysis, have shown a good correlation between formoterol administration and the relief of symptoms, dyspnea in particular.(8)
The present study was designed to investigate the immediate effect of formoterol administration in patients with poorly reversible COPD.
MethodsThis was a randomized, double-blind, placebo-controlled study involving COPD outpatients treated between January and June of 2006 at a facility specializing in lung disease. The diagnosis of COPD was made according to the criteria established by the Brazilian Thoracic Society (BTS), and all spirometric tests were conducted in accordance with BTS guidelines.(1,9) The inclusion criteria were as follows: being diagnosed with BTS stages II, III, or IV of the disease; having an FEV1 equal to or lower than 70% of predicted; and presenting no response to the short-acting bronchodilator used in the spirometric test, lack of response being defined as a variation in FEV1 of less than 200 mL, or less than 7% of predicted, at 15 min after fenoterol administration. Patients with exacerbation of symptoms were excluded, as were those with respiratory infection in the preceding four weeks, those with current or previous asthma or any other chronic pulmonary disease, as well as those who were unable to undergo plethysmography or perform the six-minute walk test.
Participating patients completed the modified Medical Research Council questionnaire,(10) designed as a means of quantifying dyspnea, which was also evaluated using the Borg scale.(11) All patients were submitted to plethysmography, after which they were randomized, in a double-blind manner, to receive, by inhalation, either 12 µg of formoterol (formoterol group) or a placebo (placebo group). The substances were presented in capsules and administered via a dry powder inhaler (AerolizerT; Boehringer Ingelheim, Ingelheim am Rheim, Germany).
The following parameters were analyzed at baseline and at 30 min after the administration of the bronchodilator or the placebo: forced vital capacity (FVC); vital capacity (VC); inspiratory capacity (IC); FEV1; FEV1/FVC ratio; total lung capacity (TLC); residual volume (RV); airway resistance (Raw); and specific airway conductance (sGaw). We used the BTS values predicted for Brazilians to calculate spirometric parameters(13) and lung volumes.(14) Briscoe & Dubois(15) predicted values were used for Raw and sGaw. The predicted values for diffusing capacity of the lung for carbon monoxide were those established by Crapo.(16)
Pulmonary function tests were performed in accordance with the BTS and the American Thoracic Society (ATS) guidelines.(12,13) The screening spirometry with bronchodilator reversibility testing was carried out using a calibrated spirometer (Koko spirometer; PDS Instrumentation Inc., Louisville, CO, USA), and plethysmography was performed using a Vmax 22 system (SensorMedics Inc., Yorba Linda, CA, USA).
On the day following plethysmography, patients performed the six-minute walk test in accordance with the ATS guidelines.(17) All patients were classified according to disease severity, following the parameters of the Body mass index, airway Obstruction, Dyspnea, and Exercise capacity (BODE) index.(18)
Data analysis was performed using a Microsoft Excel spreadsheet and the Epi Info program, version 6.04. The pre-and post-intervention (formoterol or placebo) values found for numeric variables were expressed as mean ± standard deviation, and t-tests were used for comparisons. The chi-square test was used to analyze data expressed as proportions. The level of significance was set at 5%.
The study was approved by the Ethics in Research Committee of the Porto Alegre Santa Casa Hospital, Brazil. All participating patients gave written informed consent.
ResultsForty-two consecutive adult patients were initially enrolled. Two were excluded because an accurate plethysmography measurement could not be obtained. The remaining 40 patients were randomly distributed into two groups of 20 individuals: the formoterol and the placebo groups.
The principal characteristics of the sample prior to the intervention can be seen in Table 1. All patients presented a smoking history of more than 20 pack-years, and 50% were still current smokers at the time of the study. In the plethysmographic examination, significant hyperinflation was observed in all patients (mean RV, 215% of predicted; mean TLC, 133% of predicted).
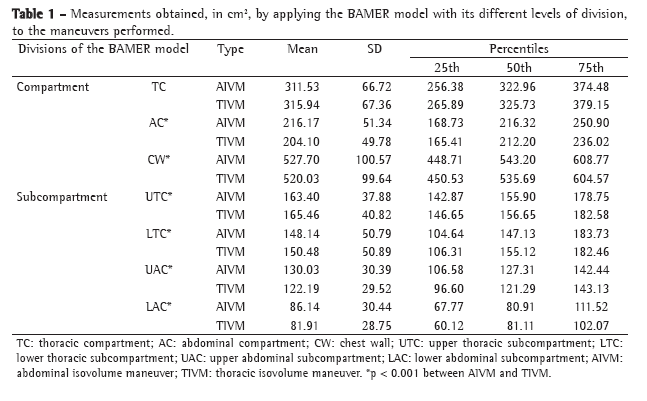
Postbronchodilator comparisons between the two groups are shown in Table 2. Functional indices and severity markers were comparable between the two groups. The mean BODE index was 2.94 in the formoterol group and 3.82 in the placebo group. There were no significant differences between the two groups in lung function measurements and dyspnea scores, although there were differences in the degree of variation of the tests when they were performed at 30 min after placebo or formoterol administration (Table 3).
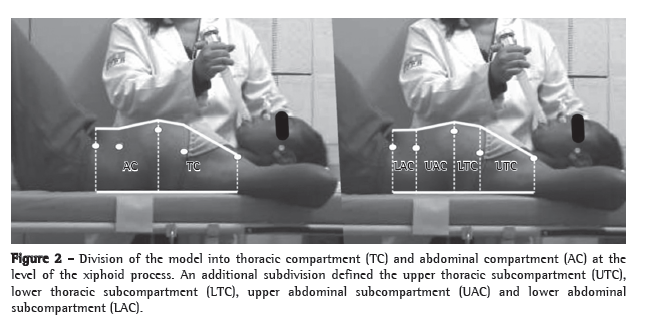

Figure 1 shows the functional variation (FEV1, FVC, IC, and Raw) at 30 min after formoterol administration. The increase in FEV1 in the formoterol group was 12.4%, compared with 0.1% in the placebo group (p = 0.00065). Similarly, the postbronchodilator increase in FVC was 12.8%, significantly greater than the 5.1% found after placebo administration (p = 0.017). The variation in IC was 7.4% in the formoterol group and −2.75% in the placebo group (p < 0.01). The considerable decrease in Raw after formoterol administration (−14%) was significantly different from the slight increase (2.6%) observed after placebo administration (p = 0.010). Although there were differences between the two groups in terms of other variables, such as VC, RV, and TLC, those differences were not significant (Figure 2).
 Discussion
DiscussionThe results of this study suggest that patients with poorly reversible COPD can present significant improvement in lung function after formoterol administration. Although formoterol is a long-acting bronchodilator, the changes were observed within 30 min after administration, which demonstrates its rapid onset of action in COPD, similar to that reported in patients with asthma.(19)
In patients with COPD, which is a heterogeneous disease, reversibility of airway obstruction might or might not occur after bronchodilator administration. Therefore, it is difficult, even when the clinical history is accurate, to determine whether the extent of reversibility is due to the characteristics of COPD or to concomitant asthma. Consequently, we chose to exclude patients who initially presented significant variability in bronchodilator reversibility testing, thereby excluding possible cases of concomitant asthma and making our sample more homogeneous. In a study involving 133 patients with asthma and 116 with COPD,(20) the mean increase in FEV1 was 307 and 120 mL , respectively, indicating that an increase in FEV1 of 200 mL is a good cut-off point to differentiate one disease from the other.
The patients included in the present study were classified as having stage II, III, or IV COPD and had an FEV1 of less than 70%-characteristics that define symptomatic patients with a greater chance to benefit from use of formoterol. In order to evaluate the severity of the disease and the homogeneity of the population under study, patients were classified according to the staging systems devised by the Global Initiative for Chronic Obstructive Lung Disease and by the BTS, as well as by applying the BODE index.(18)
In previous studies, significant FEV1 reversibility after bronchodilator use has been found in approximately one third of COPD patients.(21) However, some patients with poor reversibility after an initial inhalation of β2-agonists present better responses on subsequent tests.(22) Despite those findings, the determination of reversibility in COPD, in medical practice, is based solely on FEV1. Although the patients included in the present study were classified as poor responders to bronchodilators, 5 (25%) of those in the formoterol group presented reversibility (increased FEV1) greater than 7% of predicted and greater than 200 mL after administration of the drug. Similar results were obtained in a recent study in which little or no reversibility in bronchodilator testing was found not to be a good predictor of response to formoterol in COPD.(6) Therefore, COPD patients presenting a poor response to short-acting β2-agonists might present significant reversibility to formoterol, with considerable improvement in lung function and relief of symptoms.(4,6,24) Functional changes can be seen for weeks after the baseline testing and might be associated with several variables involved in the therapeutic efficacy of the drug.(23,24)
In a study involving 20 patients with partially reversible COPD and similar to the present study in design (formoterol and a placebo were compared), a significant response was observed even within 10 or 20 min after formoterol administration.(25) In our study, patients with stable COPD presented a substantial improvement in FEV1 at 30 min after formoterol administration, indicating that formoterol has an immediate bronchodilator effect. Other studies have indicated that formoterol administration is a safe and effective means of reversing airway obstruction in acute exacerbation of COPD.(26)
Although FEV1 remains the parameter most widely used to evaluate reversibility, the results of some studies(7,22) have indicated that FEV1 alone might not accurately determine the parallel clinical improvement of the obstruction. Dynamic hyperinflation, found in moderate to severe COPD, is more closely associated with exercise tolerance and perception of dyspnea. Studies have demonstrated, with good reproducibility, that an increase in IC after bronchodilator reversibility testing is associated with lung deflation. Therefore, IC can be used as an alternative criterion for detecting functional improvement in cases in which a variation in FEV1 is not manifest.(27) Some authors consider IC to be better than VC for that purpose.(27,28) In the present study, we found a significant difference between the formoterol group and the placebo group in terms of the variations in IC and FVC, both of which were greater after formoterol administration. Although not significant, the variation in VC was also greater in the formoterol group than in the placebo group.
One group of authors used FEV1 as a parameter to study 84 smokers with COPD in whom no variation in bronchodilator testing was observed.(29) The authors observed a improvements of 61% in RV, 40% in FVC, and 30% in slow vital capacity. In another study evaluating the effect that bronchodilators have on IC and dyspnea in COPD found significant bronchodilation and relief of symptoms at 30 min after formoterol administration, which was not observed after the administration of salmeterol or even salbutamol.(30) A significant correlation was found between an increase in IC, indicating lung deflation, and relief of dyspnea.
Although not significant, the difference between the formoterol group and the placebo group in terms of the post-test decrease in RV was substantial (−12.8 vs. −4.1%). This finding is associated with lung deflation due to the bronchodilator effect of formoterol. In the previously mentioned study of 84 patients with COPD,(29) in which irreversibility was based on FEV1, the authors found that RV decreased by 0.51 ± 0.09 L in patients with severe disease and by 0.27 ± 0.04 L in those with moderate disease. Our findings indicate that, even in patients with poorly reversible COPD, a decrease in RV can occur as soon as 30 min after formoterol administration.
A decrease in Raw was also found after formoterol administration, this variation being substantially greater than in the placebo group, thereby confirming the rapid onset of action of the drug. In another study involving patients with poorly reversible COPD,(3) a significant and immediate (within 10 min) variation in Raw was found, even when a low (6-µg) dose of formoterol was used. The authors suggested that a variation in Raw be used together with FEV1 to evaluate bronchodilator reversibility in COPD.
In the present study, a difference was found between the two groups in terms of sGaw, which increased to a greater degree in the formoterol group, although the difference was not significant. Various authors have studied the importance of sGaw in the evaluation of bronchodilator response.(3) However, the role that sGaw plays in the evaluation of bronchodilator reversibility in COPD remains unclear.
Formoterol has been recommended as one of the bronchodilators of choice in the maintenance treatment of moderate to advanced COPD.(1,2) The efficacy of its bronchodilator effect, as well as its rapid onset of action, has led some authors to suggest that it should also be used as a relief medication for symptoms in acute exacerbation of COPD.(8) Most studies have used FEV1 alone to evaluate the immediate bronchodilator effect of formoterol in patients with poorly reversible COPD, few having also evaluated parameters associated with lung hyperinflation, exercise tolerance, and dyspnea. The plethysmographic measurements reported in the present study are not routinely used in the evaluation of bronchodilator response in obstructive disease. However, it is possible that such measurements can further understanding of the bronchodilator mechanism in COPD.
Our study shows that formoterol has a rapid and efficient bronchodilator effect in patients with poorly reversible COPD. Further studies with larger samples are warranted in order to more accurately determine the extent of the immediate benefit of the drug in such patients.
References 1. Sociedade Brasileira de Pneumologia-SBPT. II Consenso Brasileiro sobre Doença Pulmonar Obstrutiva Crônica - DPOC - 2004. J Bras Pneumol. 2004;30(Supl 5):S1-S42.
2. GOLD - Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [homepage on the Internet]. Executive Summary, Global Strategy for the Diagnosis, Management, and Prevention of COPD. Updated 2005 [cited 2007 Mar 23]. Available from: http://www.goldcopd.org/Guidelineitem.asp?l1=2&l2=1&intId=1662
3. Maesen BL, Westermann CJ, Duurkens VA, van den Bosch JM. Effects of formoterol in apparently poorly reversible chronic obstructive pulmonary disease. Eur Respir J. 1999;13(5):1103-8.
4. C Cazzola M, Centanni S, Regorda C, di Marco F, di Perna F, Carlucci P, et al. Onset of action of single doses of formoterol administered via Turbuhaler in patients with stable COPD. Pulm Pharmacol Ther. 2001;14(1):41-5.
5. Donohue JF. Therapeutic responses in asthma and COPD. Bronchodilators. Chest. 2004;126(2 Suppl):125S-137S; discussion 159S-161S.
6. Muir JF, Benhamou D, Cuvelier A, Le Gros V, Overend T, Till D, et al. FEV1 reversibility does not adequately predict effect of formoterol via Aerolizer in chronic obstructive pulmonary disease. Int J Clin Pract. 2004;58(5):457-64.
7. Gimeno F, Postma DS, van Altena R. Plethysmographic parameters in the assessment of reversibility of airways obstruction in patients with clinical emphysema. Chest. 1993;104(2):467-70.
8. Bouros D, Kottakis J, Le Gros V, Overend T, Della Cioppa G, Siafakas N. Effects of formoterol and salmeterol on resting inspiratory capacity in COPD patients with poor FEV(1) reversibility. Curr Med Res Opin. 2004;20(5):581-6.
9. Pereira CA. Espirometria. J Pneumol. 2002;28(3):S1-S82.
10. Ferrer M, Alonso J, Morera J, Marrades RM, Khalaf A, Aguar MC, et al. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med. 1997;127(12):1072-9.
11. Wilson RC, Jones PW. A comparison of the visual analogue scale and modified Borg scale for the measurement of dyspnoea during exercise. Clin Sci (Lond). 1989;76(3):277-82.
12. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3): 1107-36.
13. Pereira CA, Barreto SP, Simões JG, Pereira FW, Gerstler JG, Nakatani J. Valores de referência para espirometria em uma amostra da população brasileira adulta. J Pneumol. 1992;18(1):10-22.
14. Neder JA, Andreoni S, Castelo-Filho A, Nery LE. Reference values for lung function tests. I. Static volumes. Braz J Med Biol Res. 1999;32(6):703-17.
15. Briscoe WA, Dubois AB. The relationship between airway resistance, airway conductance and lung volume in subjects of different age and body size. J Clin Invest. 1958;37(9):1279-85.
16. Crapo RO. Carbon monoxide diffusing capacity (transfer factor). Sem Respir Crit Care Med. 1998;19:335-47.
17. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-7.
18. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-12.
19. Rubin AS, Perin C, Pelegrin L, Fernandes JC, da Silva LC. Efficacy of inhaled formoterol in reversing bronchoconstriction. J Bras Pneumol. 2006;32(3):202-6.
20. Chhabra SK, Bhatnagar S. Comparison of bronchodilator responsiveness in asthma and chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2002;44(2):91-7.
21. Tavares FM, Silva LC, Rubin AS. Measuring forced expiratory volume in one second alone is not an accurate method of assessing response to bronchodilators in chronic obstructive pulmonary disease. J Bras Pneumol. 2005;31(5):407-14.
22. Cazzola M, Vinciguerra A, Di Perna F, Matera MG. Early reversibility to salbutamol does not always predict bronchodilation after salmeterol in stable chronic obstructive pulmonary disease. Respir Med. 1998;92(8):1012-6.
23. Molimard M, Bourcereau J, Le Gros V, Bourdeix I. Total reversibility testing as indicator of the clinical efficacy of formoterol in COPD. Respir Med. 2005;99(6):695-702.
24. Cazzola M, Matera MG, Santangelo G, Vinciguerra A, Rossi F, D'Amato G. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89(5):357-62.
25. Celik G, Kayacan O, Beder S, Durmaz G. Formoterol and salmeterol in partially reversible chronic obstructive pulmonary disease: A crossover, placebo-controlled comparison of onset and duration of action. Respiration. 1999;66(5):434-9.
26. Cazzola M, Santus P, Matera MG, Carlucci P, Belloli E, Di Marco F, et al. A single high dose of formoterol is as effective as the same dose administered in a cumulative manner in patients with acute exacerbation of COPD. Respir Med. 2003;97(5):458-62.
27. Connellan SJ, Gough SE. The effects of nebulized salbutamol on lung function and exercise tolerance in patients with severe airflow obstruction. Br J Dis Chest. 1982;76(2):135-42.
28. Ramsdell JW, Tisi GM. Determination of bronchodilation in the clinical pulmonary function laboratory. Role of changes in static lung volumes. Chest. 1979;76(6):622-8.
29. O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1557-65.
30. Di Marco F, Milic-Emili J, Boveri B, Carlucci P, Santus P, Casanova F, et al. Effect of inhaled bronchodilators on inspiratory capacity and dyspnoea at rest in COPD. Eur Respir J. 2003;21(1):86-94.
____________________________________________________________________________________________________________________
Study carried out in the Pavihão Pereira Filho Ward of the Porto Alegre Santa Casa Hospital, Post-Graduation Program in Pulmonology of the Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - Porto Alegre, Brazil.
1. Coordinator of the Residency in Pulmonology Program. Pavihão Pereira Filho Ward of the Porto Alegre Santa Casa Hospital, Porto Alegre, Brazil.
2. Masters student in the Postgraduate Pulmonology Program. Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - Porto Alegre, Brazil.
3. Professor in the Department of Pulmonology. Fundação Faculdade Federal de Ciências Médicas de Porto Alegre - FFFCMPA, Federal Foundation School of Medical Sciences of Porto Alegre - Porto Alegre, Brazil.
4. Professor in the Department of Internal Medicine. Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Correspondence to: Adalberto Sperb Rubin. Rua Anita Garibaldi, 1226/1403, CEP 90450-000, Porto Alegre, RS, Brasil.
Tel 55 51 3330-1813. E-mail: arubin@terra.com.br
Submitted: 23 March 2007. Accepted, after review: 4 September 2007.
**A versão completa em português deste artigo está disponível em www.jornaldepneumologia.com.br






