ABSTRACT
Objective: To identify factors associated with the minimal clinically important difference (MCID) for health-related quality of life (HRQoL) after physical conditioning in patients with COPD. Methods: Thirty-five patients were submitted to a 12-week program of physical conditioning (strength training plus low-intensity aerobic exercise). Body composition, incremental treadmill test results, endurance treadmill test results, six-minute walk test results, peripheral muscle strength, MIP, baseline dyspnea index (BDI) and Saint George's Respiratory Questionnaire (SGRQ) scores were assessed at baseline and after the program, thus allowing the variations (Δ) to be calculated. The MCID for HRQoL was defined as a reduction of ≥ 4% in the SGRQ total score. Subjects who responded to the program, achieving the MCID for HRQoL, were allocated to the responders (R) group (n = 24), and the remainder were allocated to the non-responders (NR) group (n = 11). Results: The values obtained for the following variables were significantly higher in group R than in group NR (p < 0.05): FEV1 (1.48 ± 0.54 L vs. 1.04 ± 0.34 L); VEF1/FVC (47.9 ± 11.7% vs. 35.5 ± 10.7%); PaO2 (74.1 ± 9.7 mmHg vs. 65.0 ± 8.9 mmHg); and ΔBDI, expressed as median and interquartile range (2.0 [0.0-3.5] vs. 0.0 [0.0-1.0]). The ΔBDI correlated significantly with the ΔSGRQ symptoms domain score, activity domain score and total score (r = 0.44, 0.60 and 0.62, respectively, p < 0.01 for all). After logistic regression, only ΔBDI remained as a predictor of MCID for HRQoL. Conclusions: Achieving the MCID for HRQoL after physical conditioning is associated with dyspnea reduction in COPD patients. Therefore, there is a need to develop treatment strategies designed to interrupt the dyspnea-inactivity-dyspnea cycle in such patients.
Keywords:
Pulmonary disease, chronic obstructive; Quality of life; Dyspnea; Exercise; Rehabilitation.
RESUMO
Objetivo: Investigar os fatores associados à diferença clinicamente significativa da qualidade de vida (DCSQV) após condicionamento físico em pacientes com DPOC. Métodos: Trinta e cinco pacientes foram submetidos a 12 semanas de condicionamento físico, envolvendo treinamento de força e exercício aeróbio leve. Composição corporal, teste incremental e de endurance em esteira, teste de caminhada de seis minutos, força muscular periférica, PImáx, baseline dyspnea index (BDI) e Saint George's Respiratory Questionnaire (SGRQ) foram avaliados antes e após o treinamento, e suas alterações (Δ) foram calculadas. A DCSQV foi definida como a redução ≥ 4% no escore total do SGRQ. Os pacientes que responderam ao treinamento, apresentando DCSQV, foram alocados no grupo respondedores (R; n = 24), e os demais pacientes foram alocados no grupo não-respondedores (NR; n = 11). Resultados: Os seguintes resultados foram significativamente maiores no grupo R que no grupo NR (p < 0,05): VEF1 (1,48 ± 0,54 L vs. 1,04 ± 0,34 L), VEF1/CVF (47,9 ± 11,7% vs. 35,5 ± 10,7%), PaO2 (74,1 ± 9,7 mmHg vs. 65,0 ± 8,9mmHg) e ΔBDI [mediana (interquartil); 2,0 (0,0-3,5) vs. 0,0 (0,0-1,0)]. Houve correlação significativa (p < 0,01) de ΔSGRQ-sintomas (r = 0,44), ΔSGRQ-atividade (r = 0,62) e ΔSGRQ-total (r = 0,60) com ΔBDI. Após regressão logística, apenas ΔBDI foi selecionado como determinante da DCSQV. Conclusões: A DCSQV após o condicionamento físico está associada principalmente à redução da dispneia nos pacientes com DPOC. Portanto, são necessárias estratégias de tratamento visando interromper o ciclo dispneia-sedentarismo-dispneia nesses pacientes.
Palavras-chave:
Doença pulmonar obstrutiva crônica; Qualidade de vida; Dispneia; Exercício; Reabilitação.
IntroduçãoDispneia, intolerância ao exercício e redução do estado geral de saúde são problemas comuns em pacientes com DPOC. Essas manifestações foram inicialmente atribuídas à limitação ventilatória; entretanto, a disfunção dos músculos esqueléticos periféricos, reconhecida como a principal manifestação extrapulmonar da DPOC, também limita a capacidade para realizar exercícios e está relacionada a sintomas, como fadiga e dispneia.(1) Nesse contexto, o exercício físico é recomendado como a principal intervenção dos programas de reabilitação pulmonar para pacientes com DPOC (evidência 1A).(2)
A melhora da qualidade de vida relacionada à saúde (QVRS) é um dos desfechos mais importantes após programas de reabilitação pulmonar,(3-5) e diversos instrumentos foram desenvolvidos para avaliá-la em pacientes com DPOC. O questionário de qualidade de vida Saint George's Respiratory Questionnaire (SGRQ, Questionário do Hospital Saint George na Doença Respiratória) é uma das ferramentas mais discriminativas(6) e tem versão validada para língua portuguesa e cultura brasileira.(7)
Nesse instrumento, reduções iguais ou superiores a 4% no escore total são representativas de diferença clinicamente significativa da QVRS (DCSQV).(6)
A incapacidade funcional relacionada às atividades da vida diária associada à dispneia pode ser avaliada por meio do baseline dyspnea index (BDI, índice de dispneia basal) desenvolvido por Mahler et al.(8)
Questões simples contidas nesse instrumento podem identificar o prejuízo no estado geral de saúde, e a correlação entre dispneia e QVRS está bem documentada em estudos com delineamento transversal.(9-11) Contudo, estudos com delineamento longitudinal são necessários para avaliar a correlação entre as alterações da dispneia e as alterações da QVRS após intervenções em pacientes com DPOC. Nesse sentido, o objetivo do presente estudo foi investigar os atributos clínicos e fisiológicos determinantes da DCSQV após programas de exercícios físicos em pacientes com DPOC.
MétodosForam convidados a participar do estudo 51 pacientes consecutivamente admitidos em um programa de reabilitação pulmonar. Os critérios para a inclusão foram o diagnóstico de DPOC de acordo com os critérios da Global Initiative for Chronic Obstructive Lung Disease: história clínica, exposição aos fatores de risco, relação VEF1/CVF < 70% e incremento < 15% (ou 200 mL) no VEF1 após inalação de 400 µg de salbutamol.(12) A estabilidade clínica, caracterizada pela ausência de exacerbações ou de alterações na terapia medicamentosa dentro de dois meses anteriores ao início do estudo, foram critérios de inclusão adicionais. Pacientes envolvidos em programas de reabilitação até um ano antes do início do estudo e aqueles com evidências de doenças cardiovasculares e/ou osteoarticulares foram excluídos da pesquisa. Quatro pacientes não completaram o processo inicial de avaliação e foram excluídos. Portanto, a amostra final foi composta de 47 pacientes, os quais foram randomicamente distribuídos em três tipos de programas de exercícios físicos de 12 semanas, três dias/semana. Todos os pacientes foram esclarecidos quanto aos procedimentos propostos pelo presente estudo e assinaram um termo de consentimento livre e esclarecido. O estudo foi aprovado pelo Comitê de Ética em Pesquisa do Hospital das Clínicas da Faculdade de Medicina de Botucatu.
Foram avaliados, em um espirômetro Med-Graph 1070 (Medical Graphics Corporation, St. Paul, MN, EUA) a CVF, o VEF1 e a relação VEF1/CVF, de acordo com os critérios da American Thoracic Society.(13) Os resultados da espirometria foram expressos em valores absolutos e como percentual dos valores previstos.(14) A PImáx foi avaliada de acordo com os métodos descritos por Black & Hyatt.(15) A PaO2 e a PaCO2 foram avaliadas no sangue arterial utilizando-se um analisador de gases (Stat Profile 5 Plus; Nova Biomedical, Waltham, MA, EUA). O sangue foi puncionado da artéria radial com o paciente em repouso e respirando ar ambiente.
Peso e estatura foram mensurados e o índice de massa corpórea (IMC) foi calculado em kg/m2. A impedância bioelétrica foi realizada (BIA 101A; RJL systems, Detroit, MI, EUA) para determinar a massa magra do corpo (MMC em kg) por meio de equação de regressão grupo específica.(16) O índice de MMC foi calculado (IMMC = MMC/estatura2), e a presença de depleção de MMC foi registrada quando o IMMC foi inferior a 16 kg/m2 para os homens ou inferior a 15 kg/m2 para as mulheres.(17)
A versão validada para a língua portuguesa e cultura brasileira do SGRQ foi utilizada para avaliar a QVRS dos pacientes.(7) A DCSQV foi definida como uma redução ≥ 4% no escore total do SGRQ após o treinamento. A versão traduzida(18) do BDI(8) foi utilizada para avaliar a dispneia relacionada às atividades do cotidiano no momento inicial do estudo e após as 12 semanas de condicionamento físico.
A força muscular periférica foi avaliada por meio do teste de uma repetição máxima (1RM) de acordo com as recomendações do American College of Sports Medicine.(19) Adicionalmente, a força de preensão manual da mão dominante foi avaliada por meio de um dinamômetro (TEC-60; Technical Products, Clifton, NJ, EUA).(20)
Os pacientes foram submetidos ao teste de exercício incremental limitado por sintomas em esteira rolante, utilizando-se o protocolo de Bruce modificado.(21) Em dia subsequente, foi realizado o teste de endurance com carga constante. Em resumo, após três minutos de caminhada em intensidade mínima, a esteira rolante foi regulada a 80% da velocidade e 80% da inclinação encontradas no teste incremental. Os pacientes foram instruídos a caminhar o maior tempo possível nessa carga constante. A capacidade funcional de exercício foi também avaliada por meio do teste de caminhada de seis minutos (TC6). Com exceção do formato oval do percurso de 33,12 m, todos os procedimentos do teste seguiram as recomendações da American Thoracic Society.(22)
Os pacientes foram randomicamente distribuídos para treinamento de força (TF), exercícios gerais de baixa intensidade (EGBI), ou treinamento combinado (TC = 50% TF + 50% EGBI). Os detalhes acerca dos programas de exercício, assim como seus resultados, foram previamente descritos.(23) O TF consistiu de três series de 12 repetições, com 2 min de intervalo entre as series e intensidade entre 50% e 80% da 1RM, em sete exercícios realizados em equipamentos de musculação. O EGBI foi composto de 30 min de caminhada livre com intensidade autodeterminada e 30 min de exercícios gerais de baixa intensidade realizados com pesos livres, em colchonetes e em barras paralelas. O TC envolveu 30 min de TF com duas series de 8 repetições em intensidade entre 50% e 80% da 1RM e 30 min destinados aos exercícios gerais de baixa intensidade com metade do volume do EGBI. Todos os programas de exercícios foram conduzidos em sessões de 60 min de duração com intervalos suficientes de descanso entre os exercícios.
A análise estatística foi realizada por meio do software SigmaStat 2.03 (SPSS Inc, Chicago, IL, EUA) e Systat 1.0 (Systat Software Inc., San Jose, CA, EUA). Foram utilizados os seguintes testes estatísticos: Kolmogorov-Smirnov para a análise descritiva dos dados (media ± dp), qui-quadrado ou teste exato de Fisher para comparar proporções, e coeficientes de correlação de Pearson ou Spearman para avaliar a correlação entre as variáveis estudadas.
Os pacientes que apresentaram DCSQV após o treinamento foram alocados no grupo (R), e os pacientes que não apresentaram DCSQV foram alocados no grupo (NR). Os atributos basais e suas alterações absolutas (Δ) nos grupos R e NR foram comparados por meio do teste t. A análise de regressão múltipla logística foi utilizada para avaliar os atributos associados com a DCSQV. As variáveis que apresentaram valores médios significativamente diferentes entre os grupos R e NR (p < 0,05) foram incluídas como variáveis independentes no modelo de regressão logística. Entre os atributos de função pulmonar, o VEF1, em percentual dos valores previstos, e a PaO2 foram priorizados como aqueles com maior relevância clínica em relação à CVF, VEF1/CVF e SpO2. Adicionalmente, incluímos no modelo variáveis que apresentaram valores de p < 0,2 na comparação entre os dois grupos.
ResultadosDos 47 pacientes avaliados inicialmente, 12 (4 no grupo TF, 5 no grupo EGBI e 3 no grupo TC) não completaram as 12 semanas de treinamento devido às seguintes razões: falta de motivação (n = 5); exacerbação da doença (n = 4); dificuldades socioeconômicas (n = 1); conflito com o trabalho (n = 1); e queixas musculoesqueléticas inespecíficas (n = 1). Portanto, 35 pacientes completaram os protocolos de treinamento com aderência adequada (≥ 85% das 36 sessões). Resumidamente, ao final dos programas, com exceção da força muscular periférica, que apresentou resultados mais consistentes nos grupos TF e TC, não houve nenhuma influência do tipo de exercício realizado na QVRS, na dispneia e na capacidade para realizar exercícios. Após o período de treinamento, nenhum dos grupos de treinamento apresentou alterações significativas na função pulmonar ou na composição corporal. Detalhes desses resultados estão apresentados em um artigo já publicado (23).
As características basais dos 35 pacientes estão apresentadas na Tabela 1. Vinte e quatro pacientes (68,57%) apresentaram melhora clinicamente significativa no escore total do SGRQ e foram alocados no grupo R (7 no grupo TF, 8 no grupo TC e 9 no grupo EGBI; p > 0.05). Em média, os pacientes apresentaram obstrução ao fluxo aéreo moderada. Nenhum paciente apresentou evidências de hipoxemia, definida como PaO2 < 55 mmHg e SpO2 < 89%. Três pacientes apresentaram IMC < 21 kg/m2. Outros 3 apresentaram IMMC < 15 kg/m2 (mulheres) ou < 16 kg/m2 (homens). Um paciente apresentou IMC e IMMC abaixo da normalidade.

A comparação das características basais entre os grupos R e NR antes do início do programa de condicionamento físico, mostrou que o VEF1, a VEF1/CVF e a PaO2 foram significativamente superiores no grupo R (Tabela 1). A mediana (percentil 25% - percentil 75%) da diferença na sensação de dispneia entre o início e o final do programa de reabilitação (ΔBDI) foi significativamente superior no grupo R [R = 2 (0-3,5) vs. NR = 0 (0-1); p = 0,023]. Além disso, o peso corporal (R = 71,0 ± 12,5kg vs. NR = 62,5 ± 14,12 kg; p = 0,083) e a PImáx (R = −73,4 ± 23,1 cmH2O vs. NR = −57,7 ± 23,9 cmH2O; p = 0,074) mostraram tendência de valores basais superiores no grupo R. A Figura 1 apresenta as correlações entre ΔSGRQ e ΔBDI. ΔBDI apresentou correlações positivas significativas com ΔSGRQ-sintomas, ΔSGRQ-atividade e ΔSGRQ-total. Utilizando-se ΔBDI, VEF1 (% do previsto), PaO2, peso corporal e PImáx como variáveis independentes na análise de regressão logística, apenas o ΔBDI foi selecionado como determinante da DCSQV no escore total do SGRQ (Tabela 2).
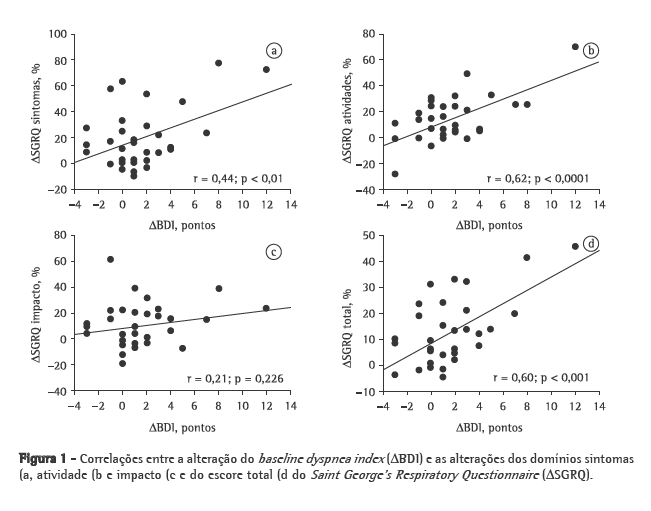
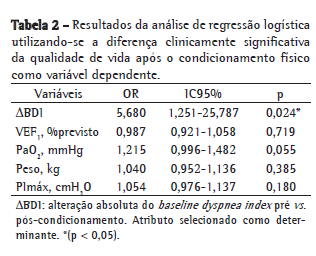 Discussão
DiscussãoAvaliamos as características e marcadores da DPOC capazes de discriminar os pacientes com ou sem melhora clinicamente significativa da qualidade de vida após programas de reabilitação pulmonar.
Encontramos dois principais resultados. Primeiro, a redução da dispneia após o condicionamento físico foi o determinante mais importante para a DCSQV. Segundo, a DCSQV ocorreu na maioria dos pacientes submetidos aos programas de condicionamento físico independentemente da composição do treinamento utilizado.
O presente estudo, em delineamento longitudinal, reforça os achados de estudos com delineamento transversal,(9,10) que destacam a dispneia como o principal atributo clínico capaz de predizer a qualidade de vida em pacientes com DPOC. No mesmo sentido, alguns autores(24) mostraram que, ao final de um programa multidisciplinar de reabilitação pulmonar, a redução da dispneia após a realização do TC6 correlacionou-se significativamente com a melhora do escore total do SGRQ. Por outro lado, outros autores(25) avaliaram os fatores basais determinantes da melhora da qualidade de vida em 53 pacientes com DPOC submetidos a um programa de reabilitação pulmonar e os acompanharam por um ano após a interrupção do programa. Nesse estudo, os autores mostraram, após uma análise de regressão logística, que apenas a PaCO2 foi selecionada como determinante da manutenção da melhora da qualidade de vida a longo prazo, após o programa de reabilitação pulmonar. Entretanto, esses autores(25) não incluíram a dispneia basal ou a alteração da dispneia no modelo de regressão logística. Nossos resultados, em consonância com os de outro estudo,(24) confirmaram a maior influência da dispneia quando comparada a outros atributos fisiológicos na qualidade de vida dos pacientes com DPOC em delineamento longitudinal. Mostramos também que o BDI pode ser um instrumento útil para quantificar a melhora da qualidade de vida após o condicionamento físico em pacientes com DPOC. Esse achado está de acordo com os de alguns autores(3) que utilizaram o BDI para avaliar a percepção de dispneia e observaram que essa ferramenta foi responsiva para quantificar a redução dos sintomas após três programas de exercícios físicos em pacientes com DPOC.
De acordo com o encontrado no presente estudo, alguns autores(26) não observaram correlações entre a melhora da qualidade de vida e a melhora da distância percorrida em teste de caminhada. Esses autores(26) submeteram pacientes com DPOC a um programa de reabilitação pulmonar e os acompanharam por um ano após o término do programa. Constataram que a melhora do tempo de endurance em cicloergômetro, diferentemente do observado para a melhora da distância percorrida em teste de caminhada, mostrou correlações com a melhora dos domínios atividade e impacto e do escore total do SGRQ após o seguimento. Em um estudo de revisão sistemática com meta-análise,(27) mostrou-se que os resultados do teste de caminhada após a reabilitação pulmonar são bastante variáveis e que pacientes com DPOC mais grave necessitam de pelo menos 6 meses de reabilitação para que a melhora da distância percorrida seja evidente, o que explica em parte a grande variabilidade dos resultados. Além disso, a capacidade de caminhar o mais rápido possível pode ser afetada por expectativas pessoais e motivação e, por isso, está mais relacionada à dispneia resultante de atividades cotidianas.(28) A melhora da tolerância ao exercício ocorre devido a adaptações neuromusculares e, sobretudo, pelo alívio da sensação de dispneia após a reabilitação,(27) o que indiretamente pode proporcionar a melhora da qualidade de vida. Portanto, a dispneia está frequentemente associada à qualidade de vida em pacientes com DPOC devido ao mecanismo de dispneia em espiral e à diminuição crônica do condicionamento, comumente apresentados por esses pacientes.(9,10)
Dados da literatura sugerem que índices espirométricos não são capazes de predizer adequadamente a qualidade de vida em pacientes com DPOC em delineamentos transversais.(28) O método tradicional de avaliar a progressão da DPOC tem sido a avaliação do declínio do VEF1(29) e, até o momento, a cessação do tabagismo é o único tratamento que reconhecidamente reduz a velocidade de declínio do VEF1 nesses pacientes.(29) Ensaios clínicos controlados randomizados com grupos placebo mostraram que tratamentos com broncodilatadores e corticosteroides são incapazes de reduzir a velocidade de declínio do VEF1 ao longo do tempo.(30) É de se esperar, portanto, que o exercício físico não resulte em alterações significativas do VEF1, e isso pode explicar em parte a ausência de correlações entre as alterações dos índices espirométricos e as alterações da qualidade de vida no presente estudo.
Observamos que o grupo R foi composto por pacientes com menor gravidade da DPOC, evidenciada por melhores índices de função pulmonar e gases arteriais. Alguns autores(27) observaram, em uma meta-análise, que os pacientes com DPOC grave e muito grave apresentam reduções significativas da dispneia somente quando envolvidos em programas de reabilitação com duração de seis meses ou mais. Em contrapartida, os pacientes com DPOC leve e moderada apresentaram reduções significativas da dispneia tanto em programas de curto prazo como em programas de seis meses ou mais. Identificamos, no presente estudo, atributos determinantes de DCSQV que devem ser considerados em intervenções de curto prazo. Nesse sentido, estudos futuros devem ser delineados para a identificação desses atributos em programas de reabilitação pulmonar de longo prazo.
O presente estudo apresenta limitações que devem ser consideradas. Tratou-se de uma análise post hoc dos dados de um estudo delineado para comparar os efeitos de três tipos de treinamento.(23) Entretanto, o cálculo amostral para o estudo original foi realizado com base na DCSQV (isto é, na redução ≥ 4% do escore total do SGRQ), indicando a necessidade de n = 10 pacientes em cada grupo de treinamento. Os resultados mostraram melhora significativa do escore total do SGRQ nos três grupos de treinamento, sem diferenças entre eles. Nesse sentido, com relação à melhora da qualidade de vida, o número de pacientes avaliados foi adequado. Além disso, os resultados estão de acordo com estudos que não mostraram diferenças significativas na melhora das principais variáveis de estado geral de saúde, sobretudo da qualidade de vida, entre diversos tipos de treinamento.(1-5,23) A presente pesquisa apresenta resultados complementares aos publicados previamente,(23) e o número de pacientes nos grupos R e NR permitiu a identificação de algumas diferenças entre as variáveis no momento basal. Embora o tamanho da amostra tenha sido pequeno, assumimos um poder estatístico de 80%.
Podemos concluir que a redução da sensação de dispneia após programas de condicionamento físico é um importante preditor da DCSQV em pacientes com DPOC. Nossos resultados reforçam a importância de estratégias de reabilitação pulmonar visando à redução desse sintoma e à interrupção do ciclo de dispneia em espiral comumente encontrado nos pacientes com DPOC. Secundariamente, observamos que o condicionamento físico isoladamente foi capaz de melhorar a qualidade de vida da maioria dos pacientes com DPOC envolvidos no presente estudo.
Referências 1. Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173(12):1390-413.
2. Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4S-42S.
3. Ortega F, Toral J, Cejudo P, Villagomez R, Sánchez H, Castillo J, et al. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(5):669-74.
4. Mador MJ, Bozkanat E, Aggarwal A, Shaffer M, Kufel TJ. Endurance and strength training in patients with COPD. Chest. 2004;125(6):2036-45.
5. Bernard S, Whittom F, Leblanc P, Jobin J, Belleau R, Bérubé C, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(3):896-901.
6. Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19(3):398-404.
7. Souza TC, Jardim JR, Jones P. Validação do Questionário do Hospital Saint George na Doença Respiratória (SGRQ) em pacientes portadores de doença pulmonar obstrutiva crônica no Brasil. J Pneumol. 2000;26(3):119-28.
8. Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751-8.
9. Mahler DA, Harver A. A factor analysis of dyspnea ratings, respiratory muscle strength, and lung function in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;145(2 Pt 1):467-70.
10. Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T, Izumi T. A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPD. Chest. 1999;116(6):1632-7.
11. Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T. Stages of disease severity and factors that affect the health status of patients with chronic obstructive pulmonary disease. Respir Med. 2000;94(9):841-6.
12. Sociedade Brasileira de Pneumologia e Tisiologia. II Consenso brasileiro sobre doença pulmonar obstrutiva crônica - DPOC. J Bras Pneumol. 2004;30(Suppl. 5):S1-S42.
13. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107-36.
14. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725-34.
15. Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99(5):696-702.
16. Kyle UG, Pichard C, Rochat T, Slosman DO, Fitting JW, Thiebaud D. New bioelectrical impedance formula for patients with respiratory insufficiency: comparison to dual-energy X-ray absorptiometry. Eur Respir J. 1998;12(4):960-6.
17. Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147(5):1151-6.
18. Martinez JAB, Padua AI. Dispnéia: novos conhecimentos sobre um velho problema. In: Sociedade Paulista de Pneumologia e Tisiologia, organizer. Pneumologia: atualização e reciclagem - volume IV. São Paulo: Vivali; 2001. p. 1-12.
19. American College of Sports Medicine. Diretrizes do ACSM para os testes de esforço e sua prescrição. Rio de Janeiro: Guanabara Koogan; 2008.
20. Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69-74.
21. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85(4):546-62.
22. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-7.
23. Dourado VZ, Tanni SE, Antunes LC, Paiva SA, Campana AO, Renno AC, et al. Effect of three exercise programs on patients with chronic obstructive pulmonary disease. Braz J Med Biol Res. 2009;42(3):263-71.
24. Katsura H, Yamada K, Wakabayashi R, Kida K. The impact of dyspnoea and leg fatigue during exercise on health-related quality of life in patients with COPD. Respirology. 2005;10(4):485-90.
25. Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, et al. Factors in maintaining long-term improvements in health-related quality of life after pulmonary rehabilitation for COPD. Qual Life Res. 2005;14(10):2315-21.
26. Laviolette L, Bourbeau J, Bernard S, Lacasse Y, Pepin V, Breton MJ, et al. Assessing the impact of pulmonary rehabilitation on functional status in COPD. Thorax. 2008;63(2):115-21.
27. Salman GF, Mosier MC, Beasley BW, Calkins DR. Rehabilitation for patients with chronic obstructive pulmonary disease: meta-analysis of randomized controlled trials. J Gen Intern Med. 2003;18(3):213-21.
28. Jones PW, Wijkstra PJ. Quality of life in patients with chronic obstructive pulmonary disease. Eur Respir Mon. 2006;38(1):375-86.
29. Mahler DA, Criner GJ. Assessment tools for chronic obstructive pulmonary disease: do newer metrics allow for disease modification? Proc Am Thorac Soc. 2007;4(7):507-11.
30. Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343(26):1902-9.
Trabalho realizado no Programa de Reabilitação Pulmonar da Faculdade de Medicina de Botucatu, Universidade Estadual Paulista, Botucatu (SP) Brasil.
Endereço para correspondência: Victor Zuniga Dourado, Departamento de Ciências da Saúde, Av. Almirante Saldanha da Gama, 89, CEP 11030-400, Santos, SP, Brasil.
Tel: 55 13 3261-3324. E-mail: vzdourado@yahoo.com.br
Apoio financeiro: Este estudo recebeu apoio financeiro do Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Recebido para publicação em 16/2/2009. Aprovado, após revisão, em 21/5/2009.
Sobre os autores Victor Zuniga Dourado
Professor Adjunto. Universidade Federal de São Paulo - UNIFESP - Campus Baixada Santista, Santos (SP) Brasil.
Letícia Cláudia de Oliveira Antunes
Fisioterapeuta. Hospital das Clínicas da Faculdade de Medicina de Botucatu - UNESP - Botucatu (SP) Brasil.
Suzana Erico Tanni
Médica Pneumologista. Hospital das Clínicas da Faculdade de Medicina de Botucatu - UNESP - Botucatu (SP) Brasil.
Irma Godoy
Professora Adjunta. Disciplina de Pneumologia, Faculdade de Medicina de Botucatu - UNESP - Botucatu (SP) Brasil.






