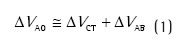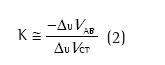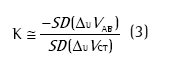ABSTRACT
Objective: To compare two methods of respiratory inductive plethysmography (RIP) calibration in three different positions. Methods: We evaluated 28 healthy subjects (18 women and 10 men), with a mean age of 25.4 ± 3.9 years. For all of the subjects, isovolume maneuver calibration (ISOCAL) and qualitative diagnostic calibration (QDC) were used in the orthostatic, sitting, and supine positions. In order to evaluate the concordance between the two calibration methods, we used ANOVA and Bland-Altman plots. Results: The values of the constant of proportionality (K) were significantly different between ISOCAL and QDC in the three positions evaluated: 1.6 ± 0.5 vs. 2.0 ± 1.2, in the supine position, 2.5 ± 0.8 vs. 0.6 ± 0.3 in the sitting position, and 2.0 ± 0.8 vs. 0.6 ± 0.3 in the orthostatic position (p < 0.05 for all). Conclusions: Our results suggest that QDC is an inaccurate method for the calibration of RIP. The K values obtained with ISOCAL reveal that RIP should be calibrated for each position evaluated.
Keywords:
Plethysmography; Respiratory mechanics; Posture; Calibration.
RESUMO
Objetivo: Comparar dois métodos de calibração da pletismografia respiratória por indutância (PRI) em três posturas diferentes. Métodos: Foram avaliados 28 indivíduos saudáveis (18 mulheres e 10 homens), com média de idade de 25,4 ± 3,9 anos. Todos os indivíduos foram submetidos a isovolume maneuver calibration (ISOCAL, calibração por manobra de isovolume) e qualitative diagnostic calibration (QDC, calibração diagnóstica qualitativa) em ortostatismo, sedestação e decúbito dorsal. Foi utilizada ANOVA e a disposição gráfica de Bland-Altman para a avaliação da concordância dos métodos de calibração. Resultados: Os valores da constante de proporcionalidade (K) foram significativamente distintos entre ISOCAL e QDC nas três posturas avaliadas: 1,6 ± 0,5 vs. 2,0 ± 1,2, em decúbito dorsal; 2,5 ± 0,8 vs. 0,6 ± 0,3, em sedestação; e 2,0 ± 0,8 vs. 0,6 ± 0,3, em ortostatismo (p < 0,05 para todos). Conclusões: Nossos resultados sugerem que QDC não é um método acurado para a calibração da PRI. Os valores de K obtidos por ISOCAL mostram que a PRI deve ser calibrada para cada postura avaliada.
Palavras-chave:
Pletismografia; Mecânica respiratória; Postura; Calibragem.
IntroduçãoNas últimas décadas, considerável atenção tem sido direcionada para os dispositivos de mensuração não invasiva da parede torácica.(1,2) Dentre os dispositivos mais empregados para avaliar a configuração e a coordenação toracoabdominal, destaca-se a pletismografia respiratória por indutância (PRI), fundamentada em 1967 por Konno e Mead, que propuseram um modelo matemático para viabilizar a avaliação quantitativa dos movimentos relacionados à ventilação espontânea.(3) A equação do modelo proposto é:
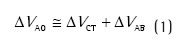
onde ∆VAO é a mudança de volume medido na via aérea, ∆VCT é a mudança de volume na caixa torácica, e ∆VAB é a mudança de volume no abdome.(3,4)
Os equipamentos de PRI são geralmente compostos por duas faixas elásticas transdutoras, que são posicionadas na caixa torácica e no abdome. Essas faixas geram sinais elétricos proporcionais à movimentação desses compartimentos; por isso, devem ser calibradas previamente para a correta avaliação do sistema respiratório. Dentre os diversos métodos empregados para a calibração dos equipamentos de PRI, destacam-se isovolume maneuver calibration (ISOCAL, calibração por manobra de isovolume),(3) considerada o padrão ouro, e qualitative diagnostic calibration (QDC, calibração qualitativa diagnóstica).(4)
Na ISOCAL, o indivíduo é orientado a trocar voluntariamente o volume entre a caixa torácica e o abdome com as vias aéreas ocluídas. Essa manobra respiratória faz com que o valor de ∆VAO seja zero, e por isso estabelece a seguinte equação:
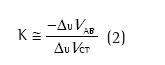
onde K estima a contribuição relativa dos sinais elétricos relacionados com a caixa torácica (∆UVCT) e com o abdome (∆UVAB), sendo esses sinais não calibrados (U).(2-7)
A QDC é realizada durante um determinado intervalo de tempo de respiração basal e utiliza uma aproximação estatística da equação 2 para determinar K sem a realização de manobras respiratórias. A equação que rege o QDC é:
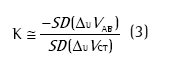
onde SD(∆UVAB) e SD(∆UVCT) são os desvios-padrão dos sinais elétricos relativos ao abdome e à caixa torácica, respectivamente.(4,8,9)
Apesar de a ISOCAL ser considerada o padrão ouro para a calibração da PRI, a grande maioria dos estudos emprega a QDC, devido ao fato de que essa não demanda manobras respiratórias específicas.(10-13) Desconhecemos, até o presente momento, estudos comparando os valores da constante K dos métodos de calibração da PRI quando diferentes posturas são empregadas.
MétodosFoi avaliada uma amostra de conveniência composta por 28 voluntários adultos (18 mulheres e 10 homens) sem histórico prévio de doenças e com idades variando entre 18 e 65 anos. Os critérios de exclusão foram ser tabagista, ter diagnóstico de doenças respiratórias, ter dificuldade de compreensão para a realização das manobras respiratórias, apresentar deformidade da caixa torácica, ter doença osteomuscular que influenciasse a mecânica respiratória e apresentar alterações espirométricas. O comitê de ética em pesquisa da instituição aprovou o estudo (protocolo 1221/09), e todos os voluntários assinaram o termo de consentimento livre e esclarecido.
A sequência de realização dos métodos de calibração e das posturas foi estabelecida de maneira aleatória, por sorteio. O protocolo do estudo foi composto pelas seguintes etapas: obtenção de dados antropométricos (peso e altura), utilizando uma balança mecânica calibrada (Welmy S.A., Santa Bárbara do Oeste, Brasil); realização de provas de função pulmonar (Quark PFT; Cosmed, Roma, Itália); calibração dos sinais elétricos emitidos pelas cintas; calibração do fluxo a ser quantificado pelo pneumotacógrafo; posicionamento das duas cintas elásticas indutoras, adaptadas ao tamanho do indivíduo, no tórax e no abdome; aleatorização da sequência de aplicação dos métodos de calibração (ISOCAL ou QDC); e aleatorização da sequência de aplicação das posturas (decúbito dorsal, sedestação e ortostatismo).
O aparelho para PRI (Respitrace; NIMS Inc., Miami, FL, EUA) utilizado em nosso estudo é composto por duas faixas elásticas transdutoras (Inductotrace System, Ambulatory Monitoring, Ardsley, NY, EUA), que possuem diversas numerações adaptáveis a cada indivíduo, um oscilador e um sistema de aquisição e condicionamento de sinais analógicos. O equipamento foi acoplado ao sistema de um conversor analógico-digital (USB 1208LS, Measurement Computing, Norton, MA, EUA) de 12 bits que trabalha com uma frequência de amostragem de 50 Hz para a realização de uma análise digital dos dados obtidos. Um pneumotacógrafo Fleisch número 2 (Hugo Sachs Elektronik, Freiburg, Alemanha) e um transdutor de pressão diferencial (modelo HCXPMQ05D6V; Sensortechnics, Puchheim, Alemanha) foram inseridos para a monitorização do fluxo aéreo e agregados ao equipamento de PRI. Para evitar que o vapor d'água do ar exalado se condensasse no interior do pneumotacógrafo, um sistema de aquecimento presente no pneumotacógrafo foi alimentado com 6 V em corrente alternada. Um coletor de saliva também foi adaptado entre o pneumotacógrafo e o bocal. O sinal elétrico analógico gerado pelo transdutor de pressão diferencial foi amplificado utilizando um sistema eletrônico com amplificador de instrumentação (modelo AD620; Analog Devices, Norwood, MA, EUA).
Os sinais de fluxo, assim como as variações de volumes do tórax e do abdome, foram integrados e digitalizados em ambiente LabVIEW, versão 8.5 (National Instruments, Austin, TX, EUA). Os sinais podiam ser verificados em tempo real, permitindo iniciar ou interromper a gravação dos dados adquiridos para sua posterior análise. O processamento dos sinais foi realizado em ambiente MATLAB (The Mathworks, Natick, MA, EUA), que permitia a detecção dos ciclos respiratórios dos três sinais disponíveis, assim como o cálculo dos parâmetros respiratórios e dos valores da constante de proporcionalidade dos sinais elétricos dos compartimentos (K). Os valores de K foram obtidos, utilizando-se ISOCAL e QDC, a partir dos dados referentes à manobra respiratória de isovolume e ao intervalo de tempo de coleta de 5 min, respectivamente.
Na ISOCAL, os voluntários foram orientados sobre a forma de execução da manobra respiratória exigida por esse método de calibração, com as vias aéreas ocluídas com um clipe nasal,(3) objetivando evitar o efeito de aprendizado previamente à gravação dos sinais elétricos. Na QDC, os voluntários foram orientados a respirar tranquilamente pelo bocal do pneumotacógrafo por um intervalo de tempo de coleta de 5 min.(4) Para garantir a oclusão das vias aéreas superiores, o clipe nasal também foi utilizado.
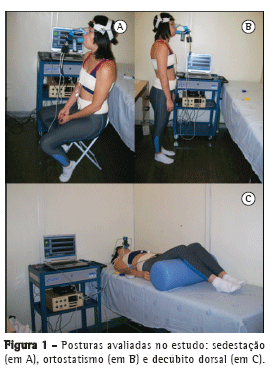
Foram feitas calibrações para cada método e para cada uma das três posturas estudadas. No decúbito dorsal, o indivíduo foi posicionado horizontalmente sem elevação do decúbito e com o quadril e os joelhos fletidos; na sedestação, os voluntários foram posicionados em uma cadeira sem apoio dorsal, com angulação de 90° no quadril e no joelho; no ortostatismo, os voluntários foram orientados a adotar uma postura ereta, com os braços relaxados ao longo do corpo, e manter a distribuição do peso corporal de forma simétrica nos membros inferiores (Figura 1).
A normalidade dos dados foi avaliada pelo teste de Kolmogorov-Smirnov, e os dados foram expressos em medianas (dados não paramétricos) ou médias e respectivos intervalos de confiança (dados paramétricos). Para a comparação dos parâmetros avaliados, foi utilizada ANOVA para medidas repetidas, e o nível de significância foi ajustado para 5% (p < 0,05). A disposição gráfica de Bland-Altman foi utilizada para avaliar a concordância entre os dois métodos de calibração (Figura 2). Os programas SigmaStat, versão 9.1 (Systat Software Inc., San Jose, CA, EUA) e MedCalc (MedCalc Software, Mariakerke, Bélgica) foram empregados nas análises estatísticas.
 Resultados
ResultadosA caracterização antropométrica dos participantes do estudo está apresentada na Tabela 1.

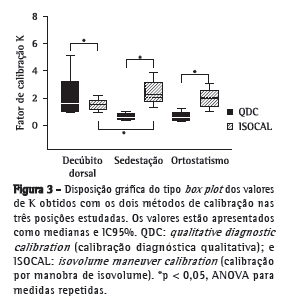
A avaliação das calibrações foi feita pela análise comparativa da constante de proporcionalidade (K). Os valores da constante K estão apresentados na Figura 3, e pode-se verificar que os valores obtidos com o método QDC foram diferentes daqueles obtidos com o método ISOCAL nas três posturas avaliadas. Em decúbito dorsal, os valores de K obtidos com QDC foram significativamente superiores aos valores com ISOCAL (2,2 ± 1,6 vs. 1,50 ± 0,47; p < 0,05). Em contrapartida, em sedestação e em ortostatismo, os valores de K obtidos com QDC foram significativamente inferiores aos valores com ISOCAL (0,6 ± 0,3 vs. 2,4 ± 0,9 e 0,6 ± 0,3 vs. 1,9 ± 0,7; p < 0,05 para ambos). Não foi observada diferença entre os valores de K com QDC em decúbito dorsal e aqueles com ISOCAL em ortostatismo.
Além disso, a representação gráfica de Bland-Altman mostra a não concordância entre os métodos de calibração nas diferentes posturas avaliadas. No gráfico, pode-se observar que a diferença entre os dois métodos aumenta ao longo do eixo x, ou seja, com o aumento dos valores médios das duas calibrações (Figura 2).
Em relação à contribuição dos compartimentos torácico e abdominal, foi possível verificar que, nas três posturas avaliadas, houve uma maior contribuição da caixa torácica em relação ao abdome, pois os valores de K obtidos com ISOCAL foram maiores que 1,0 nas posições decúbito dorsal, sedestação e ortostatismo (1,5 ± 0,4; 2,4 ± 0,9; e 1,9 ± 0,7, respectivamente). A inserção do sistema de monitoração do fluxo permitiu a avaliação acurada das variáveis respiratórias, coletadas a partir da respiração basal (Tabela 2).
 Discussão
DiscussãoO presente estudo mostrou que, independentemente do método de calibração, os valores de K diferiram entre as posturas, sugerindo que deve ser realizada uma calibração para cada postura a ser avaliada. Além disso, foi verificado que o método QDC apresentou resultados distintos daqueles obtidos por ISOCAL, independentemente da posição avaliada, sugerindo que a sua obtenção não pode ser considerada acurada.
A PRI tem sido amplamente utilizada; porém, devido à dificuldade em se realizar o padrão ouro (ISOCAL), diversos estudos têm sugerido a utilização do método QDC.(11-14) Uma das grandes vantagens do método QDC é a facilidade da sua realização, pois, nesse método, o indivíduo necessita apenas manter a respiração basal durante 5 min, e a calibração é feita pela variação obtida entre os resultados de cada compartimento. Essa metodologia pode ser questionável frente ao princípio de calibração da PRI, que é a sua variação frente a mudanças de volume entre a caixa torácica e o abdome.(3) Além disso, o método QDC pressupõe a manutenção de um padrão respiratório do indivíduo ao longo do intervalo de tempo de medida, o que parece não ter acontecido durante a coleta dos nossos resultados.
A hipótese de Sackner et al.(4) para a utilização do QDC consiste no conceito de que esse tipo de calibração requer um padrão respiratório constante para que seja possível a determinação de K. Entretanto, nossos achados mostram que ocorreu uma grande dispersão dos tempos de ciclos respiratórios, associados a valores elevados de desvios-padrão do volume corrente durante o ciclo respiratório basal (Tabela 2). Esses dados sugerem que, durante a coleta de dados para a realização da QDC, o padrão respiratório não foi constante e, portanto, sugerem que 5 min durante a respiração tranquila para a aquisição dos dados podem não ser suficientes para instituir o valor de K referente à contribuição dos compartimentos. Nesse sentido, o estudo realizado por Sartene et al.(7) demonstrou que, durante a respiração tranquila, ocorre uma dispersão de aproximadamente 20% do volume corrente médio, e apenas 8% desse valor corresponde à contribuição dos compartimentos. Por essa razão, os autores afirmam que o emprego de calibrações que demandem respiração tranquila é questionável quando essa é empregada em dispositivos com dois graus de liberdade, uma vez que a maioria dos participantes do estudo, quando avaliados em decúbito dorsal, utilizaram majoritariamente somente um grau de liberdade.
Os achados de diferenças entre as calibrações observadas no nosso estudo são corroborados pelo estudo de Thompson,(15) no qual também foram observadas diferenças nos valores da constante K entre os dois métodos de calibração. Naquele estudo, Thompson sugere que a QDC apresenta limitações importantes referentes ao cálculo de K, pois se considera que a soma da leitura dos sinais é constante para os compartimentos da caixa torácica e do abdome e que a participação de cada compartimento é obtida pela subtração dessa estimativa. De Groote et al.(8) demonstraram que a diminuição da variabilidade na contribuição dos movimentos do tórax e do abdome pode gerar um fator de calibração impreciso na condição em que ocorrem medidas com respirações em que o volume corrente é mantido constante ou quase constante, como pressupõe o método QDC.
O método QDC em crianças também não se mostrou confiável para a estimativa do volume corrente.(11) Segundo os autores, as contribuições da caixa torácica e do abdome de crianças anestesiadas não foram suficientes para permitir a derivação exata de K. Um resultado observado no presente estudo foi a contribuição da caixa torácica para os volumes correntes obtidos, que foram inesperadamente elevados. Por outro lado, Adams et al.(14) relataram a obtenção de valores de K satisfatórios quando a QDC foi realizada em recém-nascidos. Contudo, os autores não avaliaram a contribuição relativa dos compartimentos e, consequentemente, a acurácia dos valores de K obtidos.
Nosso estudo apresenta algumas possíveis limitações. As medições foram realizadas com os voluntários respirando pela boca, utilizando um pneumotacógrafo, e esse tipo de respiração foi assegurado porque eles utilizavam um clipe nasal. Isso pode ter modificado o fluxo de ar inspirado e o padrão respiratório. Apesar disso, em nosso estudo, não foi observada uma alteração significativa do padrão respiratório com a inserção do sistema de monitorização do fluxo ao sistema de PRI. Acreditamos que a escolha do pneumotacógrafo do tipo Fleisch, considerado um transdutor de fluxo gasoso de baixa resistência, possa ter contribuído positivamente para o padrão respiratório observado.
Apesar de o método ISOCAL ser considerado pouco atrativo do ponto de vista prático, ele ainda é considerado o padrão ouro para a calibração da PRI.(16) No nosso estudo, tentamos assegurar a qualidade dos dados obtidos orientando os voluntários quanto à realização da manobra para ISOCAL antes mesmo da sua realização. Portanto, uma possível alternativa para o emprego desse método de calibração seria a orientação do indivíduo antes da sua execução. A nossa hipótese é que mesmo o método ISOCAL sofre influências da posição corporal. Temos ciência de somente um estudo que demonstrou que mudanças de postura, principalmente nas transições de ortostatismo para sedestação, levam a alterações no coeficiente volume-movimento, que podem influenciar expressivamente a acurácia do método de calibração e, consequentemente, a estimativa do volume obtido pela PRI.(17)
Os resultados do nosso estudo foram similares aos observados por Zimmerman et al.,(17) visto que foram verificadas diferenças entre as posturas avaliadas até mesmo na calibração considerada como padrão ouro (ISOCAL), e essas diferenças foram ainda mais acentuadas nas transições de ortostatismo para sedestação (Figura 3). Os nossos resultados sugerem a necessidade de se realizar a calibração quando a PRI é empregada para a estimativa dos volumes toracoabdominais em um mesmo indivíduo a cada postura avaliada.
Em relação à participação dos compartimentos para a ventilação, foi possível observar uma maior contribuição da caixa torácica para o volume corrente nas três posturas (Figura 3), visto que os valores médios da constante K (relação da participação torácica/abdominal) obtidos com o método ISOCAL foram superiores a 1,0 nas posições decúbito dorsal, sedestação e ortostatismo. Esse resultado é corroborado pelo estudo de Verschakelen & Demedts,(16) que demonstraram que existe uma maior contribuição da caixa torácica nas posturas mais altas, como sedestação e ortostatismo. De acordo com os mesmos autores, mulheres apresentam uma maior contribuição da caixa torácica principalmente nas posturas mais altas quando comparadas aos homens. No presente estudo, houve um predomínio de mulheres (65,2%), o que também poderia explicar os valores de K obtidos nas duas posturas. Além disso, os movimentos da caixa torácica e do abdome dependem do estado de contração dos músculos respiratórios, e a contribuição do diafragma para a movimentação desses compartimentos depende da ação da gravidade. Portanto, a participação do abdome para a ventilação será maior em ortostatismo do que em sedestação devido à ação hidráulica do conteúdo abdominal, que favorece uma maior complacência do abdome.(18) Os dados obtidos em nosso estudo confirmam essa hipótese, pois, apesar de haver uma maior contribuição da caixa torácica nas duas posturas, o valor de K obtido para ortostatismo (1,9 ± 0,7) foi menor do que o obtido para sedestação (2,4 ± 0,9). Acreditamos que ocorre uma maior participação do abdome em ortostatismo do que em sedestação, apesar de a movimentação ser prioritariamente torácica.
Embora nossos resultados sejam considerados satisfatórios em relação à aplicabilidade da ISOCAL para a calibração da PRI, um sistema para a monitorização da pressão do ar aprisionado na boca durante a realização da ISOCAL não foi acoplado ao equipamento. Isso pode ser considerado uma grande limitação do estudo, pois não foi possível verificar quantitativamente se houve compressão de ar na boca dos pacientes.(19) Por outro lado, mesmo que de forma qualitativa, foi observado que os voluntários não aprisionaram ar na boca, pois esses não distendiam suas bochechas.
Os dados obtidos no presente estudo sugerem que a QDC não pode ser considerada um método acurado para a calibração da PRI e que ISOCAL deve ser realizada para cada postura avaliada.
AgradecimentosOs autores agradecem aos pesquisadores José António Fernandes e Liane Hirano Shiguemichi a sua colaboração.
Referências1. Banovcin P, Seidenberg J, von der Hardt H. Pressure sensor plethysmography: a method for assessment of respiratory motion in children. Eur Respir J. 1995;8(1):167-71. PMid:7744184. http://dx.doi.org/10.1183/09031936.95.08010167
2. Brüllmann G, Fritsch K, Thurnheer R, Bloch KE. Respiratory monitoring by inductive plethysmography in unrestrained subjects using position sensor-adjusted calibration. Respiration. 2010;79(2):112-20. PMid:19365103. http://dx.doi.org/10.1159/000212117
3. Konno K, Mead J. Measurement of the separate volume changes of rib cage and abdomen during breathing. J Appl Physiol. 1967;22(3):407-22. PMid:4225383.
4. Sackner MA, Watson H, Belsito AS, Feinerman D, Suarez M, Gonzalez G, et al. Calibration of respiratory inductive plethysmograph during natural breathing. J Appl Physiol. 1989;66(1):410-20. PMid:2917945.
5. De Groote A, Wantier M, Cheron G, Estenne M, Paiva M. Chest wall motion during tidal breathing. J Appl Physiol. 1997;83(5):1531-7. PMid:9375316.
6. Kondo T, Uhlig T, Pemberton P, Sly PD. Laser monitoring of chest wall displacement. Eur Respir J. 1997;10(8):1865-9. PMid:9272932. http://dx.doi.org/10.1183/09031936.97.10081865
7. Sartene R, Dartus C, Bernard JL, Mathieu M, Goldman MD. Comparison of thoracoabdominal calibration methods in normal human subjects. J Appl Physiol. 1993;75(5):2142-50. PMid:8307871.
8. De Groote A, Wantier M, Cheron G, Estenne M, Paiva M. Chest wall motion during tidal breathing. J Appl Physiol. 1997;83(5):1531-7. PMid:9375316.
9. Mayer OH, Clayton RG Sr, Jawad AF, McDonough JM, Allen JL. Respiratory inductance plethysmography in healthy 3- to 5-year-old children. Chest. 2003;124(5):1812-9. PMid:14605053. http://dx.doi.org/10.1378/chest.124.5.1812
10. Warren RH, Horan SM, Robertson PK. Chest wall motion in preterm infants using respiratory inductive plethysmography. Eur Respir J. 1997;10(10):2295-300. PMid:9387956. http://dx.doi.org/10.1183/09031936.97.10102295
11. Brown K, Aun C, Jackson E, Mackersie A, Hatch D, Stocks J. Validation of respiratory inductive plethysmography using the Qualitative Diagnostic Calibration method in anaesthetized infants. Eur Respir J. 1998;12(4):935-43. PMid:9817172. http://dx.doi.org/10.1183/09031936.98.12040935
12. Miller C, Hoffman AM, Hunter J. Thoracoabdominal asynchrony failed to grade airway obstructions in foals. J Appl Physiol. 2000;88(6):2081-7. PMid:10846021.
13. Panka GF, Oliveira MM, França DC, Parreira VF, Britto RR, Velloso M. Ventilatory and muscular assessment in healthy subjects during an activity of daily living with unsupported arm elevation. Rev Bras Fisioter. 2010;14(4):337-44. PMid:20949234. http://dx.doi.org/10.1590/S1413-35552010005000013
14. Adams JA, Zabaleta IA, Stroh D, Johnson P, Sackner MA. Tidal volume measurements in newborns using respiratory inductive plethysmography. Am Rev Respir Dis. 1993;148(3):585-88. http://dx.doi.org/10.1164/ajrccm/148.3.585
15. Thompson JR. Qualitative diagnostic calibration technique. J Appl Physiol. 1999;87(2):869-72. PMid:10484687.
16. Verschakelen JA, Demedts MG. Normal thoracoabdominal motions. Influence of sex, age, posture, and breath size. Am J Respir Crit Care Med. 1995;151(2 Pt 1):399-405. PMid:7842198.
17. Zimmerman PV, Connellan SJ, Middleton HC, Tabona MV, Goldman MD, Pride N. Postural changes in rib cage and abdominal volume-motion coefficients and their effect on the calibration of a respiratory inductance plethysmograph. Am Rev Respir Dis. 1983;127(2):209-14. PMid:6830037.
18. Agostini EP, Mognoni P, Torri G, Saracino F. Relation between changes of rib cage circumference and lung volume. J Appl Physiol. 1965;20(6):1179-86.
19. Gonzalez H, Haller B, Watson HL, Sackner MA. Accuracy of respiratory inductive plethysmograph over wide range of rib cage and abdominal compartmental contributions to tidal volume in normal subjects and in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1984;130(2):171-4. PMid:6465670.
* Trabalho realizado no Departamento de Fisioterapia e no Laboratório de Engenharia Biomédica, Universidade de São Paulo, São Paulo (SP) Brasil.
Endereço para correspondência: Celso R. F. Carvalho. Faculdade de Medicina da Universidade de São Paulo, Avenida Dr. Arnaldo, 455, sala 1210, CEP 01246-903, São Paulo, SP, Brasil.
Tel. 55 11 3061-7317. Fax: 55 11 3085-0992. E-mail: cscarval@usp.br
Apoio financeiro: Nenhum.
Recebido para publicação em 3/6/2011. Aprovado, após revisão, em 14/2/2012.
Sobre os autoresRenata Cléia Claudino Barbosa
Mestranda em Ciências da Reabilitação, Faculdade de Medicina, Universidade de São Paulo, Brasil, e Professora Assistente, Centro Universitário São Camilo, São Paulo (SP) Brasil.
Celso Ricardo Fernandes de Carvalho
Professor Associado. Departamento de Fisioterapia, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
Henrique Takachi Moriya
Professor Assistente. Laboratório de Engenharia Biomédica, Escola Politécnica, Universidade de São Paulo, São Paulo (SP) Brasil.