ABSTRACT
Objetivo: Medir a resistência de vias aéreas utilizando a técnica de resistência do interruptor (Rint) em pacientes com fibrose cística (FC) e correlacioná-la com parâmetros espirométricos, assim como avaliar a acurácia de Rint para determinar a resposta das vias aéreas a um broncodilatador. Métodos: Estudo transversal com 38 crianças e adolescentes com FC acompanhados no Ambulatório de FC do Hospital São Lucas, em Porto Alegre (RS). Após a determinação de Rint, os pacientes foram submetidos à espirometria. Para a avaliação da resposta ao broncodilatador, as medições foram repetidas após o uso de salbutamol inalatório. Resultados: Houve uma forte correlação entre o inverso de Rint e VEF1 (r = 0,8; p < 0,001) e moderadas correlações entre o inverso de Rint e FEF25-75% (r = 0,74; p < 0,001) e entre o inverso de Rint e índice de massa corpórea (r = 0,62; p < 0,001). A curva ROC foi utilizada na comparação da resposta ao broncodilatador determinada por Rint com aquela determinada por valores espirométricos. Para um ponto de corte de −28% para Rint, a área sob a curva foi de 0,75, com uma sensibilidade de 66% e uma especificidade de 82%. Conclusões: Nossos achados indicam que Rint apresenta uma boa correlação com parâmetros espirométricos, embora a técnica Rint não tenha sido suficientemente acurada para substituir a espirometria na avaliação da resposta ao broncodilatador.
Keywords:
Testes de função respiratória; Fibrose cística; Espirometria; Resistência das vias respiratórias.
RESUMO
Objetivo: Medir a resistência de vias aéreas utilizando a técnica de resistência do interruptor (Rint) em pacientes com fibrose cística (FC) e correlacioná-la com parâmetros espirométricos, assim como avaliar a acurácia de Rint para determinar a resposta das vias aéreas a um broncodilatador. Métodos: Estudo transversal com 38 crianças e adolescentes com FC acompanhados no Ambulatório de FC do Hospital São Lucas, em Porto Alegre (RS). Após a determinação de Rint, os pacientes foram submetidos à espirometria. Para a avaliação da resposta ao broncodilatador, as medições foram repetidas após o uso de salbutamol inalatório. Resultados: Houve uma forte correlação entre o inverso de Rint e VEF1 (r = 0,8; p < 0,001) e moderadas correlações entre o inverso de Rint e FEF25-75% (r = 0,74; p < 0,001) e entre o inverso de Rint e índice de massa corpórea (r = 0,62; p < 0,001). A curva ROC foi utilizada na comparação da resposta ao broncodilatador determinada por Rint com aquela determinada por valores espirométricos. Para um ponto de corte de −28% para Rint, a área sob a curva foi de 0,75, com uma sensibilidade de 66% e uma especificidade de 82%. Conclusões: Nossos achados indicam que Rint apresenta uma boa correlação com parâmetros espirométricos, embora a técnica Rint não tenha sido suficientemente acurada para substituir a espirometria na avaliação da resposta ao broncodilatador.
Palavras-chave:
Testes de função respiratória; Fibrose cística; Espirometria; Resistência das vias respiratórias.
IntroductionPulmonary function tests are performed as part of the routine follow-up evaluation of patients with cystic fibrosis (CF). In particular, spirometry is often used in order to track disease progression and measure treatment response, FEV1 being the parameter that is most widely used. However, spirometry requires the active cooperation of patients, and this is a major limitation, especially in young children.(1,2)
Modern technology has led to the development of new equipment to measure pulmonary function and, consequently, new evaluation parameters, such as airway resistance.(3-5) Recently, the interrupter resistance (Rint) technique, which was first described in 1927 by Von Neergaard & Wirz,(6) has been increasingly used because of the portability and low cost of the new equipment and because tests can be easily performed with the technique. Resistance measurements are obtained during spontaneous ventilation and do not require any forced maneuvers.(6) The Rint technique measures airway resistance on the basis of the assumption that, after a brief (millisecond-long) airflow interruption (by the closure of a shutter) during spontaneous breathing, the pressure in the mouth and the pressure in the alveoli achieve an instantaneous balance. The method assumes that there is only one alveolar pressure value. The user-friendliness of the technique and the portability of the equipment, as well as the possibility of a correlation between Rint and spirometric values, have increased the interest in the method and its use in children and adolescents with pulmonary disease.(3,7-14) Many patients with CF use bronchodilators to relieve their symptoms or prior to physical therapy, and the measurement of bronchodilator response is an important part of the follow-up of such patients. In studies involving asthma patients, the Rint technique was shown to be accurate in measuring such a response.(15-19) The objectives of the present study were to measure airway resistance with the Rint technique in children and adolescents with CF and to determine whether the values obtained correlated with spirometric parameters, as well as to evaluate the accuracy of the Rint technique in determining the airway response to a bronchodilator.
MethodsThis was a cross-sectional study. Patients were eligible for inclusion in the study if they were aged 5-18 years and were being followed at the Cystic Fibrosis Outpatient Clinic of the Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS, Pontifical Catholic University of Rio Grande do Sul) São Lucas Hospital, located in the city of Porto Alegre, Brazil. All of the CF diagnoses had been confirmed by sweat test results or by the finding of two CF gene mutations, as well as by the clinical evaluation performed by the attending team. Patients were recruited between March of 2008 and August of 2009. Patients with pulmonary exacerbations, those using antibiotics other than those used for maintenance therapy, and those who were unable to understand the test were excluded.
A portable MicroRint® device (Micro Medical Ltd., Rochester, United Kingdom) was used in order to measure Rint, which was estimated by flow and pressure measurements obtained immediately before and during brief (100 ms) airway occlusion. The interruption of airflow occurs during the expiratory phase, at tidal volume. The data needed in order to calculate Rint were obtained by a high frequency pressure transducer (2,000 Hz), a rapidly closing valve (10 ms) for occlusion, and a pneumotachograph. The results were shown on a display device coupled to a printer. The pressure during occlusion was estimated by linear back extrapolation from a point at which the pressure had stabilized and reached a plateau.
For reasons of hygiene and in order to prevent saliva from entering the pneumotachograph, the measurements were performed with a commercially available disposable Rint filter (Micro Medical Ltd.), as recommended.
The measurements were performed at the Cystic Fibrosis Outpatient Clinic of the PUCRS São Lucas Hospital, on the day of the follow-up visit. The individuals selected for inclusion and their parents or legal guardians received information on the test and gave written informed consent. The measurements were performed in the same room where the spirometric maneuvers were performed.
Initially, height and body mass index were measured by a clinical nutritionist, with the participants barefoot and wearing light clothes. A scale (Filizola, São Paulo, Brazil) and a stadiometer were used. Age was calculated from the birth date. The patients had to be at rest for at least 10 min.
Before the test was performed, the patients were informed that noises would occur because of the closure of the shutter and were asked to remain comfortably seated and maintain quiet, spontaneous ventilation. As soon as there were no more questions regarding the execution of the test, a nose clip was placed, and the patients were instructed to close their lips around the disposable mouthpiece (2.5 cm in diameter) and place their tongues under the mouthpiece in order to prevent airflow obstruction. The face and chin were supported by the hand of the investigator in order to prevent loss of energy and reduce the effect of upper airway complacency. The head was held in a neutral position.
Before the beginning of the test, the investigator demonstrated the maneuver in order to provide clarification and familiarize the patients with it. Ten airflow interruptions were performed sequentially at unforced PEF, in ten random respiratory cycles. After the test, the Rint values and their medians were printed. The measurement was considered successful if at least five adequate measurements were obtained and if the coefficient of variation of those measurements was ≤ 20%. In situations in which the coefficient of variation was > 20%, extreme measurements were excluded until at least five remained; otherwise, the test was considered unsuccessful and was therefore excluded. The measurements were performed at a random frequency, determined automatically (i.e., independent of the investigator). Although the patients were able to hear the shutter closing, they were unable to predict when the measurement was taken.
During the measurements, leaks around the mouthpiece, neck movements, vocal fold closure, irregular breathing patterns, movements during the closure of the shutter, swallowing, and sneezing were all avoided. If any of those occurred, the measurement was rejected. The Rint values were normalized to Z scores, in accordance with local reference equations.(3)
After the measurement of airway resistance, the patients underwent spirometry as routinely done in outpatient follow-up. All of the spirometric tests were performed by the same trained resident physician, who used a Koko spirometer (PDS Instrumentation Inc., Louisville, CO, USA). Spirometric and Rint measurements were performed again 20 min after inhaled bronchodilator use (albuterol, 400 µg).
The study was approved by the Research Ethics Committee of the PUCRS São Lucas Hospital.
Variables with normal distribution were expressed as means and standard deviations, whereas those with non-normal distribution were expressed as medians and interquartile ranges (IQRs). Pearson's correlation test was used in order to evaluate correlations. In order to compare the Rint and spirometric values in terms of the bronchodilator response, a ROC curve was used, and the cut-off points were calculated by the Youden index. The data were analyzed with the Statistical Package for the Social Sciences, version 14.0 (SPSS Inc., Chicago, IL, USA).
Considering a type I error of 0.05, a power of 90%, and a correlation coefficient of 0.5 between Rint and FEV1 as clinically significant, we estimated that a sample size of 38 patients was required.(20)
ResultsBetween March of 2008 and August of 2009, 38 patients were evaluated. The mean age of the patients under study was 10.79 years, and males predominated (57.89%). The mean percentage of predicted FEV1 (FEV1%) was 82.82%, indicating mild pulmonary function impairment, and the median Z score for Rint was 0.29 (IQR: −0.32 to 0.93), indicating slightly increased Rint (Tables 1 and 2).
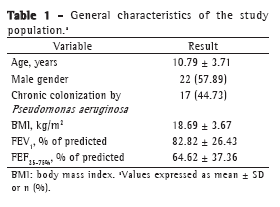
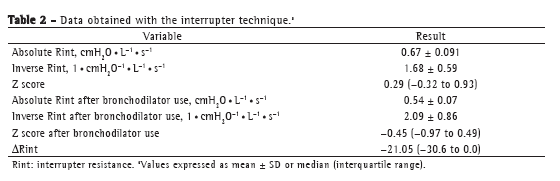
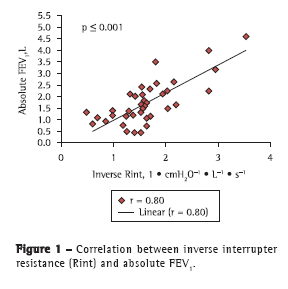
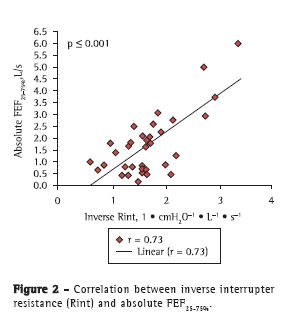
The correlations of the spirometric variables (absolute FEV1, FEV1%, absolute FEF25-75%, and percentage of predicted FEF25-75%) with the Rint values were determined by absolute and inverse correlations, as well as by Z scores. Strong correlations were found between inverse Rint (which is a measure of airway conductance) and absolute FEV1 (r = 0.80; p < 0.001), as well as between inverse Rint and absolute FEF25-75% (r = 0.73; p < 0.001), and there was a weak correlation between inverse Rint and FEV1% (r = 0.37; p < 0.001; Figures 1 and 2). In addition, there was a correlation between inverse Rint and body mass index (r = 0.62; p < 0.001).
The ROC curve was used in order to compare the bronchodilator response, as determined by Rint, with spirometric values. The area under the curve was 0.75, and, using the Youden index, we chose a cut-off point of −28%, with a sensitivity of 66% and a specificity of 82%, in order to estimate a bronchodilator response of 12%, as measured by spirometry. Therefore, of the patients in whom spirometry revealed bronchodilator response, 66% had been identified by the Rint technique, 34% of the results having been false negatives; of the patients in whom spirometry revealed no bronchodilator response, 82% had been identified by the Rint technique, 18% of the results having been false positives (Figure 3).
 Discussion
DiscussionIn the present study, Rint was evaluated in a population of CF individuals followed at the Cystic Fibrosis Outpatient Clinic of the PUCRS São Lucas Hospital, which is a relatively new referral center for the treatment of the disease, having been inaugurated in 2004. The center provides outpatient treatment to young individuals with mild pulmonary function impairment. It should be pointed out that the characteristics of our study population were similar to those of the CF population in North America, where most CF patients were found to have a mean FEV1 of 76.1% of predicted (values being normal up to age 6 years), 51.7% were found to be male, and 53.7% were found to be under 18 years of age.(21)
In our study sample, Rint was found to be slightly increased in comparison with local reference data.(3) In the present study, we analyzed the correlations between the main spirometric parameters and Rint. We also proposed a Rint cut-off point for evaluating bronchodilator response, comparing it with well-established data on the variation of FEV1. We found a significant correlation between Rint and absolute FEV1. In addition, the correlation between inverse Rint and FEV1 was found to be even stronger, which is probably due to the fact that both are flow-dependent. However, after the data were normalized, we found no significant correlation between the two, which suggests that Rint and FEV1 reflect different aspects of pulmonary function.
Our findings are similar to those reported in a study evaluating children and adolescents with CF.(17) In that study, moderate correlations were found between inverse Rint and FEV1, as well as between FVC and FEF25-75%. However, no bronchodilator response was found when the Rint technique was used.(17) In addition, data analysis in that study was different from that in the present study, in which a ROC curve was used and various cut-off points for bronchodilator response were evaluated, a Rint cut-off point of −28% having been found to be the best cut-off point to determine bronchodilator response. The accuracy of the method was reasonable, with a sensitivity of 66% and a specificity of 82%. However, spirometric values cannot be ignored, given that 34% of the results might be false negatives when bronchodilator response is evaluated by the Rint technique. A recent study reported that adult patients in whom spirometry revealed bronchodilator response also showed decreased Rint and decreased airway resistance by plethysmography, with no significant difference between the values.(22)
One limitation of the present study is that most of the patients presented with mild pulmonary function impairment, meaning that our findings should not be extrapolated to patients with disease that is more severe. This is in accordance with the results reported by Gritti & Barreto,(22) who found the agreement between Rint and airway resistance by plethysmography to be better in patients with mild pulmonary function impairment. In addition, one group of authors(23) showed that the Rint technique tends to underestimate airway resistance in obstructed children with asthma and in those with CF, and that this tendency appears to increase in proportion to the severity of airway obstruction.
In conclusion, Rint was shown to correlate well with spirometric parameters and to be reasonably accurate in evaluating the bronchodilator response in patients with mild pulmonary function impairment. Because the Rint technique requires equipment that is inexpensive, portable, and easy to use, further studies involving a greater number of patients should be conducted in order to determine whether Rint can be used as an outcome measure in follow-up studies and therapeutic trials.
References1. Sociedade Brasileira de Pneumologia e Tisiologia. Diretrizes para Testes de Função Pulmonar. J Pneumol. 2002;28(3):S1-S238
2. Ramsey BW, Boat TF. Outcome measures for clinical trials in cystic fibrosis. Summary of a Cystic Fibrosis Foundation consensus conference. J Pediatr. 1994;124(2):177-92. http://dx.doi.org/10.1016/S0022-3476(94)70301-9
3. Rech VV, Vidal PC, Melo Júnior HT, Stein RT, Pitrez PM, Jones MH. Airway resistance in children measured using the interrupter technique: reference values. J Bras Pneumol. 2008;34(10):796-803. PMid:19009212.
4. Sly PD, Lombardi E. Measurement of lung function in preschool children using the interrupter technique. Thorax. 2003;58(9):742-4. PMid:12947127. PMCid:1746798. http://dx.doi.org/10.1136/thorax.58.9.742
5. Beydon N. Interrupter resistance: what's feasible? Paediatr Respir Rev. 2006;7 Suppl 1:S5-7. PMid:16798594. http://dx.doi.org/10.1016/j.prrv.2006.04.022
6. Neergaard KV, Wirz K. Die Messung der Strömungswiderstände in den Atemwege des Menschen, insbesondere bei Asthma und Emphysema. Z Klin Med. 1927;105:21-7.
7. Phagoo SB, Watson RA, Silverman M, Pride NB. Comparison of four methods of assessing airflow resistance before and after induced airway narrowing in normal subjects. J Appl Physiol. 1995;79(2):518-25. PMid:7592212.
8. Kessler V, Mols G, Bernhard H, Haberthür C, Guttmann J. Interrupter airway and tissue resistance: errors caused by valve properties and respiratory system compliance. J Appl Physiol. 1999;87(4):1546-54. PMid:10517790.
9. McKenzie SA, Chan E, Dundas I, Bridge PD, Pao CS, Mylonopoulou M, et al. Airway resistance measured by the interrupter technique: normative data for 2-10 year olds of three ethnicities. Arch Dis Child. 2002;87(3):248-51. PMid:12193444. PMCid:1719236. http://dx.doi.org/10.1136/adc.87.3.248
10. Carter ER, Stecenko AA, Pollock BH, Jaeger MJ. Evaluation of the interrupter technique for the use of assessing airway obstruction in children. Pediatr Pulmonol. 1994;17(4):211-7. PMid:8208590. http://dx.doi.org/10.1002/ppul.1950170402
11. Hadjikoumi I, Hassan A, Milner AD. Effects of respiratory timing and cheek support on resistance measurements, before and after bronchodilation in asthmatic children using the interrupter technique. Pediatr Pulmonol. 2003;36(6):495-501. PMid:14618641. http://dx.doi.org/10.1002/ppul.10384
12. Beydon N, Amsallem F, Bellet M, Boulé M, Chaussain M, Denjean A, et al. Pulmonary function tests in preschool children with cystic fibrosis. Am J Respir Crit Care Med. 2002;166(8):1099-104. PMid:12379554. http://dx.doi.org/10.1164/rccm.200205-421OC
13. Beydon N, Amsallem F, Bellet M, Boule M, Chaussain M, Denjean A, et al. Pre/postbronchodilator interrupter resistance values in healthy young children. Am J Respir Crit Care Med. 2002;165(10):1388-94. PMid:12016101. http://dx.doi.org/10.1164/rccm.2011082
14. Beydon N, Pin I, Matran R, Chaussain M, Boulé M, Alain B, et al. Pulmonary function tests in preschool children with asthma. Am J Respir Crit Care Med. 2003;168(6):640-4. PMid:12869361. http://dx.doi.org/10.1164/rccm.200303-449OC
15. Bridge PD, Lee H, Silverman M. A portable device based on the interrupter technique to measure bronchodilator response in schoolchildren. Eur Respir J. 1996;9(7):1368-73. PMid:8836645. http://dx.doi.org/10.1183/09031936.96.09071368
16. Phagoo SB, Wilson NM, Silverman M. Evaluation of a new interrupter device for measuring bronchial responsiveness and the response to bronchodilator in 3 year old children. Eur Respir J. 1996;9(7):1374-80. PMid:8836646. http://dx.doi.org/10.1183/09031936.96.09071374
17. Davies PL, Doull IJ, Child F. The interrupter technique to assess airway responsiveness in children with cystic fibrosis. Pediatr Pulmonol. 2007;42(1):23-8. PMid:17106902. http://dx.doi.org/10.1002/ppul.20523
18. Black J, Baxter-Jones AD, Gordon J, Findlay AL, Helms PJ. Assessment of airway function in young children with asthma: comparison of spirometry, interrupter technique, and tidal flow by inductance plethysmography. Pediatr Pulmonol. 2004;37(6):548-53. PMid:15114556. http://dx.doi.org/10.1002/ppul.20046
19. Kannisto S, Vanninen E, Remes K, Korppi M. Interrupter technique for evaluation of exercise-induced bronchospasm in children. Pediatr Pulmonol. 1999;27(3):203-7. http://dx.doi.org/10.1002/(SICI)1099-0496(199903)27:3%3C203::AID-PPUL9%3E3.0.CO;2-G
20. Hulley SB, Duncan MS, Schmidt MI, Duncan BB, editors. Delineando a pesquisa clínica uma abordagem epidemiológica. Porto Alegre: Artmed; 2008.
21. Cystic Fibrosis Foundation [homepage on the Internet]. Bethesda: Cystic Fibrosis Foundation [cited 2010 Mar 4]. Annual Data Report 2008. [Adobe Acrobat document, 16p.]. Available from: http://www.cff.org/UploadedFiles/aboutCFFoundation/AnnualReport/2008-Annual-Report.pdf
22. Gritti LA, Barreto SS. A new approach to the determination of airway resistance: interrupter technique vs. plethysmography. J Bras Pneumol. 2011;37(1):61-8. PMid:21390433. http://dx.doi.org/10.1590/S1806-37132011000100010
23. Oswald-Mammosser M, Charloux A, Donato L, Albrech C, Speich JP, Lampert E, et al. Interrupter technique versus plethysmography for measurement of respiratory resistance in children with asthma or cystic fibrosis. Pediatr Pulmonol. 2000;29(3):213-20. http://dx.doi.org/10.1002/(SICI)1099-0496(200003)29:3%3C213::AID-PPUL10%3E3.0.CO;2-N
* Study carried out under the auspices of the Graduate Program in Child and Adolescent Health, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Correspondence to: Alessandra Rocha. Rua Lilia Russovski Tessler, 130, Protásio Alves, CEP 91260-250, Porto Alegre, RS, Brasil.
Tel. 55 51 9275-9490. E-mail: pilates.ale@gmail.com
Financial support: This study received financial support from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development).
Submitted: 25 July 2011. Accepted, after review: 26 December 2011.
** A versão completa em português deste artigo está disponível em www.jornaldepneumologia.com.br
About the authorsAlessandra Rocha
Graduate Student. Graduate Program in Child and Adolescent Health, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Márcio Vinícius Fagundes Donadio
Professor. Pontifícia Universidade Católica do Rio Grande do Sul - PUCRS, Pontifical Catholic University of Rio Grande do Sul - School of Nursing, Nutrition, and Physiotherapy, Porto Alegre, Brazil.
Dariana Vale de Ávila
Undergraduate Student. Pontifícia Universidade Católica do Rio Grande do Sul - PUCRS, Pontifical Catholic University of Rio Grande do Sul - Porto Alegre, Brazil.
Patricia Xavier Hommerding
Professor. Centro Universitário Franciscano - UNIFRA, Franciscano University Center - Santa Maria, Brazil.
Paulo José Cauduro Marostica
Professor. Federal University of Rio Grande do Sul School of Medicine; and Group Coordinator. Assistance for Cystic Fibrosis Patients, Pontifícia Universidade Católica do Rio Grande do Sul - PUCRS, Pontifical Catholic University of Rio Grande do Sul - São Lucas Hospital, Porto Alegre, Brazil.






