ABSTRACT
Objective: To evaluate whether anthropometric and dietary intake indicators are predictors of pulmonary function in cystic fibrosis (CF) patients. Methods: This was a cross-sectional study involving 69 patients (age range, 5.4-16.5 years) diagnosed with CF under follow-up at the Hospital de Clínicas de Porto Alegre, located in the city of Porto Alegre, Brazil. Anthropometric assessment was based on body mass index (BMI), mid-arm muscle circumference (MAMC), and triceps skinfold thickness (TST). Dietary intake was assessed by using recall data, which were compared with the recommended dietary allowances. Pulmonary function was assessed by ventilatory capacity, expressed as FEV1. Prevalence ratios for the outcome studied (FEV1 < 80% of predicted) were calculated by indicator. Results: In patients with MAMC and TST below the 25th percentile, the prevalence of FEV1 < 80% of predicted was significantly higher than in those with higher MAMC and TST (p < 0.001 and p = 0.011, respectively). In comparison with other patients, those with a BMI below the 50th percentile showed a 4.43 times higher prevalence of FEV1 < 80% of predicted (95% CI: 1.58-12.41), and that prevalence was 2.54 times higher in those colonized with methicillin-resistant Staphylococcus aureus (MRSA) than in those not so colonized (95% CI: 1.43-4.53). The association between dietary intake and the prevalence of FEV1 < 80% of predicted was of only borderline significance (95% CI: 0.95-3.45). Conclusions: Not being colonized with MRSA and having a BMI above the 50th percentile appear to preserve pulmonary function in CF patients.
Keywords:
Cystic fibrosis; Respiratory function tests; Nutrition assessment; Energy intake.
RESUMO
Objetivo: Avaliar se indicadores antropométricos e de ingestão alimentar são preditores da função pulmonar em pacientes com fibrose cística (FC). Métodos: Estudo transversal com 69 pacientes (variação, 5,4-16,5 anos de idade) diagnosticados com FC e em acompanhamento no Hospital de Clínicas de Porto Alegre, em Porto Alegre (RS). A avaliação antropométrica consistiu nas medidas do índice de massa corpórea (IMC), da circunferência muscular do braço (CMB) e da dobra cutânea tricipital (DCT). A ingestão alimentar foi avaliada pelo recordatório de ingestão habitual e comparada com recommended dietary allowances. A avaliação da função pulmonar foi realizada através da capacidade ventilatória, representada pelo VEF1. Razões de prevalência foram calculadas entre os preditores e o desfecho estudado (VEF1 < 80% do previsto). Resultados: Os pacientes com CMB e DCT abaixo do percentil 25 apresentaram significativamente maior prevalência de VEF1 < 80% do previsto (p < 0,001 e p = 0,011, respectivamente). Os pacientes com IMC menor que o percentil 50 apresentaram 4,43 vezes (IC95%: 1,58-12,41) a prevalência de VEF1 < 80% do previsto. Os pacientes colonizados por Staphylococcus aureus resistente a meticilina apresentaram 2,54 vezes (IC95%: 1,43-4,53) a prevalência do desfecho do que os não colonizados. A associação entre consumo calórico e o desfecho estudado apresentou significância limítrofe (IC95%: 0,95-3,45). Conclusões: O IMC superior ao percentil 50 e a ausência de colonização por S. aureus resistente a meticilina apresentaram uma associação direta com função pulmonar preservada em pacientes com FC.
Palavras-chave:
Fibrose cística; Testes de função respiratória; Avaliação nutricional; Ingestão de energia.
IntroductionCystic fibrosis (CF) is an autosomal recessive genetic disease that affects various organs and systems, especially the respiratory and digestive tracts.(1) Lung disease is the major factor responsible for morbidity and mortality in CF patients.(2)
The chronic malnutrition and the impaired growth observed in CF patients result from an energy deficit, which is due to increased losses, and from the energy expenditure, which exceeds the dietary intake.(3) Anorexia due to respiratory and gastrointestinal complications contributes to the worsening of this profile, leading to reduced energy intake.(4)
Adequate dietary intake is essential to maintaining good nutritional status in CF patients, especially when they have pancreatic insufficiency. To that end, it is recommended that there be an intake of 120-150% of the energy requirements of healthy individuals of the same gender and age.(5,6)
The nutritional status of the patient has an important relationship with the progression of lung disease, affecting quality of life and survival.(7) Maintaining adequate nutritional status is essential for the integrity of the respiratory system in CF patients.(8,9) Stallings et al.(10) found a direct association between pulmonary function and nutritional status, demonstrating that having a body mass index (BMI) above the 50th percentile is directly associated with having an FEV1 > 80%.
The objective of the present study was to evaluate whether anthropometric and dietary intake indicators are predictors of pulmonary function in CF patients under follow-up at a referral hospital.
MethodsThis was a cross-sectional study involving 69 children and adolescents diagnosed with CF in accordance with the Cystic Fibrosis Foundation diagnostic criteria.(11) The inclusion criteria were as follows: being 18 years of age; having a confirmed diagnosis of CF (by genetic testing, sweat testing, or both); having undergone pulmonary function tests; and being under follow-up at the Pediatric Pulmonology Outpatient Clinic of the Hospital de Clínicas de Porto Alegre (HCPA, Porto Alegre Hospital de Clínicas), located in the city of Porto Alegre, Brazil. We excluded patients with pulmonary exacerbation. This paper is part of a study that is aimed at validating a nutritional screening tool for CF patients (approved by the Graduate Research Group of the HCPA; Protocol nº. 09637).
Data were collected after written informed consent was obtained during a nutritional consultation, between March and October of 2010. Anthropometric measurements included weight, height, triceps skinfold thickness (TST), and mid-arm muscle circumference (MAMC). The details of the method employed for taking the anthropometric measurements and the assessment of dietary intake (recall data) are described in Pereira et al.(12) We calculated BMI and height-for-age percentiles, in accordance with the WHO criteria,(13) as well as MAMC and TST percentiles, in accordance with the criteria established by Frisancho.(14) Data on mutation type, bacterial colonization, albumin levels, and pulmonary function-the last two items being related to the last annual checkup-were obtained from online medical records.
Pulmonary function was assessed by spirometric measurements of ventilatory capacity, expressed as FEV1, in the Pulmonology Department of the HCPA. The spirometric technique used followed the Brazilian Thoracic Association Guidelines for Pulmonary Function Testing.(15) Overall disease severity was assessed by the Shwachman-Kulczycki score, obtained by summing the scores for each of four items (general activity, physical examination, nutritional status, and radiological findings), as assessed by the attending physician and the staff nutritionist. After the scoring, the status of the patient was classified from excellent (score, 86-100) to severe (score, 40).(16)
Serum albumin was measured by the bromocresol green method during the annual checkup of the patients, and the results were collected from the medical records. Regarding bacterial colonization, the data extracted from the clinical records and related to sputum samples collected over the preceding 12 months revealed the presence of the following strains: Staphylococcus aureus; methicillin-resistant S. aureus (MRSA); Pseudomonas aeruginosa; mucoid P. aeruginosa; and Burkholderia cepacia.
The outcome analyzed in this study was FEV1 < 80% of predicted, because values above this cut-off point represent preserved pulmonary function. Anthropometric and body composition variables, as well as serum albumin levels, dietary intake, and bacterial colonization, were evaluated as possible predictors of this outcome.
Data were analyzed with the Statistical Package for the Social Sciences, version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Categorical variables are expressed as absolute and relative frequencies. Sample characteristics represented by categorical variables were tested for associations by Pearson's chi-square test. Continuous variables are expressed as mean and standard deviation. In order to compare means, we used the Student's t-test for independent samples. In order to build the adjusted model, we used Poisson regression with robust variance, progressively including variables showing a value of p < 0.2 in the univariate analysis. Variables with a value of p < 0.05 remained in the final model. The level of significance was set at 5% (p < 0.05).
ResultsWe studied 69 patients (age range, 5.4-16.5 years), 35 (50.7%) of whom were female. Age at diagnosis ranged from 0 years (infants in the neonatal period) to 12 years, the median age being 6 months. Genetic testing was performed in 63 patients (91.3%), of whom 15 (21.7%) were homozygous for the F508 mutation and 32 (46.4%) were heterozygous for the F508 mutation.
Pancreatic insufficiency was present in 63 patients (91.3%), and CF-related diabetes mellitus was present in 2 (2.9%). Only 3 patients (4.3%) in the sample were using enteral nutritional therapy (gastrostomy). Mean Shwachman-Kulczycki scores were satisfactory (> 71.0%) in 55 patients (79.7%). Table 1 shows the characteristics of the sample by nutritional and clinical parameters, as well as by pulmonary function and bacterial colonization data.
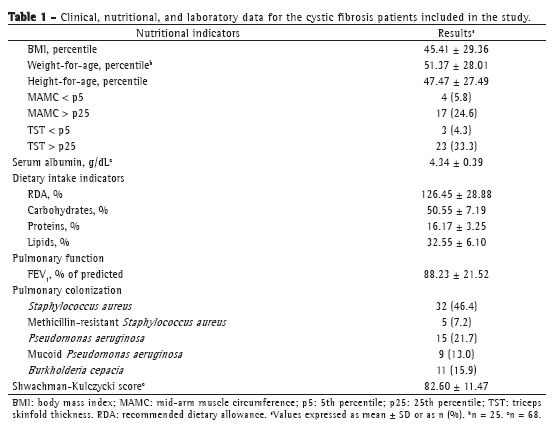
Analysis of the sample for the outcome studied revealed that patients with an FEV1 < 80% had concomitant lower nutritional parameters (Table 2). Patients with preserved pulmonary function (FEV1 > 80%) were, on average, 2.8 years younger than were those with an FEV1 < 80%. Patients with more severe pulmonary impairment had lower mean serum albumin levels, although their serum albumin levels were within the normal range. With regard to dietary intake, we found that, on average, patients with an FEV1 < 80% did not meet the CF dietary recommendation of > 120% of the recommended dietary allowance (RDA).
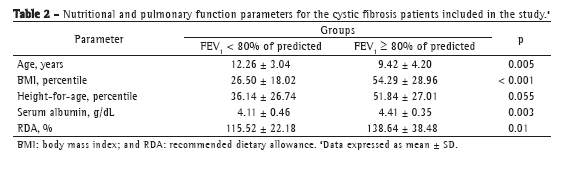
Analysis of body composition as a predictor of FEV1 demonstrated that, in patients with MAMC and TST below the 25th percentile, the prevalence of FEV1 < 80% was significantly higher than in those with higher MAMC and TST (p < 0.001 and p = 0.011, respectively). Analysis of bacterial colonization revealed no statistically significant differences among colonized patients in terms of FEV1 < 80%, except for those colonized with MRSA, in whom the prevalence of FEV1 < 80% was higher (p = 0.017).
Patients with a BMI below the 50th percentile showed a 4.43 times higher prevalence of FEV1 < 80% than did those with a BMI above the 50th percentile, the model being adjusted for age, %RDA < 120%, albumin, height-for-age percentile, MAMC < the 25th percentile, TST < the 25th percentile, and colonization with MRSA (Table 3). Colonization with MRSA was significantly associated with the outcome studied. The prevalence of FEV1 < 80% was 2.54 times higher in those colonized with MRSA than in those not so colonized, the model being adjusted for the other predictor variables. The association between dietary intake and pulmonary function was of only borderline significance.
 Discussion
DiscussionThe results of the present study support the recommendations in the literature, i.e., anthropometric measurements above the 50th percentile as a protective factor against pulmonary impairment in CF patients.(10) In addition, colonization with MRSA, which is a cause for concern among CF patients in Brazil, was found to be likewise associated with pulmonary impairment.
Our sample was found to be in good nutritional and clinical status, the values for these parameters being similar to those found in CF referral centers in developed countries.(17) The mean BMI percentile found in the present study was similar to that reported by the Cystic Fibrosis Foundation, i.e., 48.7.(17) The mean FEV1 found in our sample was equivalent to that used for classifying lung disease as mild, being similar to the mean FEV1 in the USA (76.3%); the median diagnosis was also similar.(17)
Analysis of the nutritional parameters, compared with the outcome studied (FEV1 < 80% of predicted), demonstrated that BMI, height, and %RDA values were low in our study sample. Patients with an FEV1 > 80% had concomitant mean BMI and height percentiles above the 50th percentile, whereas patients with an FEV1 < 80% had mean BMI percentiles close to the cut-off point for nutritional risk for CF patients (BMI < the 25th percentile). Chaves et al. found a statistically significant association of BMI and TST with the degree of pulmonary impairment (FEV1 < 70%).(18)
Patients with an FEV1 < 80% had a dietary intake that was lower than that recommended for CF patients, unlike patients with better pulmonary function values, who met the nutritional recommendations for CF patients. Simon et al.(19) demonstrated that 51.7% of CF patients met the recommendations of 120% of the RDA and that those patients had a median BMI percentile of 56, whereas the patients whose intake was below the recommendations had a median BMI percentile of 34.9. In another study, which assessed dietary intake in a group of CF patients in the 6-9 year age bracket, the median dietary intake was 115% of the RDA, and only 39% met the dietary recommendations.(20)
In the present study, patients with a lower mean age had preserved pulmonary function (FEV1 > 80%); this finding is in agreement with the literature, which indicates that FEV1 decreases with age, especially from adolescence onward.(10,17) Döring et al.(21) reported a progressive reduction in pulmonary function in CF patients, at an estimated rate of 1-2% per year; however, it is clear that this rate can vary according to the frequency and severity of pulmonary exacerbations.
Despite being within the normal range, albumin levels were significantly different in the group of patients with an FEV1 < 80%. Albumin is a potent antioxidant that can be essential for maintaining pulmonary glutathione levels(22,23) and has been related to lung disease severity, the prognosis for CF patients being consequently poorer.(24,25)
Analysis of body composition in this group revealed that patients with worse pulmonary function values had a concomitant higher prevalence of muscle mass and fat mass below the 25th percentile. Pedreira et al.(26) found a positive association between lean body mass and FEV1 in CF patients in the 7-17 year age bracket.
The indicator of nutritional status used in the final model, i.e., a BMI < the 50th percentile, was found to be a strong predictor of pulmonary function in CF patients, meaning that these patients showed a nearly five times higher prevalence of FEV1 < 80% in comparison with those with a BMI > the 50th percentile. Stallings et al.(10) showed that having an FEV1 close to or higher than 80% was directly associated with having a BMI the 50th percentile.
The outcome analyzed in this study, however, cannot be explained solely by impaired nutritional status. Dietary intake and bacterial colonization are other factors that affect the outcome studied. Poisson regression analysis with robust variance, adjusted for age, BMI percentile, albumin levels, height-for-age percentile, MAMC < the 25th percentile, TST < the 25th percentile, and colonization with MRSA, revealed that a dietary intake of less than 120% of the RDA was of borderline significance in predicting deterioration of pulmonary function. This was due to low sample power. However, colonization with MRSA was found to be strongly associated with the outcome studied. Dasenbrook et al.(27) observed an association between colonization with MRSA and poorer survival in CF patients. Among patients colonized with MRSA, the mortality rate was 27.7 deaths per 1,000 patient-years, whereas, among those not so colonized, the mortality rate was 18.3 deaths per 1,000 patient-years. The attributable risk percentage of death associated with MRSA was 34%.
The limitations of the present study are related to its cross-sectional design, which does not allow us to establish a causal relationship, meaning that the association between the factors studied and the prevalence of FEV1 < 80% of predicted might be subject to reverse causality. The association between adequacy of dietary intake and the outcome studied was not significant, because of a lack of study power. In this case, we would need a minimum of 100 patients per category of dietary adequacy (RDA) in order to detect an association with the outcome studied. The present study sample has a power of only 28% to measure this association. However, the present study is relevant because findings in CF patients in Brazil corroborated those in CF patients worldwide and because it underscores the importance of effective nutritional care and prevention of MRSA colonization.
The present study allows us to conclude that having a BMI above the 50th percentile and not being colonized with MRSA appear to preserve pulmonary function in CF patients.
References1. Cardoso AL, Gurmini J, Spolidoro JVN, Nogueira RJN. Nutrição e fibrose cística. Rev Bras Nutr Clin. 2007;22(2):146-54.
2. Wagener JS, Headley AA. Cystic fibrosis: current trends in respiratory care. Respir Care. 2003;48(3):234-45; discussion 246-7.
3. Wood LG, Gibson PG, Garg ML. Circulating Markers To Assess Nutritional Therapy In Cystic Fibrosis. Clin Chim Acta. 2005;353(1-2):13-29. http://dx.doi.org/10.1016/j.cccn.2004.11.002
4. Ludwig Neto N, editor. Fibrose cística: enfoque multidisciplinar. Florianópolis: Secretaria de Estado de Saúde; 2008.
5. Sinaasappel M, Stern M, Littlewood J, Wolfe S, Steinkamp G, Heijerman HG, et al. Nutrition in patients with cystic fibrosis: a European Consensus. J Cyst Fibros. 2002;1(2):51 75. http://dx.doi.org/10.1016/S1569-1993(02)00032-2
6. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academy Press; 2005.
7. Koletzko S, Reinhardt D. Nutritional challenges of infants with cystic fibrosis. Early Hum Dev. 2001;65 Suppl:S53 61. http://dx.doi.org/10.1016/S0378-3782(01)00206-7
8. Peterson ML, Jacobs DR Jr, Milla CE. Longitudinal changes in growth parameters are correlated with changes in pulmonary function in children with cystic fibrosis. Pediatrics. 2003;112(3 Pt 1):588-92. http://dx.doi.org/10.1542/peds.112.3.588
9. Stapleton D, Kerr D, Gurrin L, Sherriff J, Sly P. Height and weight fail to detect early signs of malnutrition in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2001;33(3):319-25. http://dx.doi.org/10.1097/00005176-200109000-00017
10. Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H; Clinical Practice Guidelines on Growth and Nutrition Subcommittee, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832-9. http://dx.doi.org/10.1016/j.jada.2008.02.020
11. Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4 S14. http://dx.doi.org/10.1016/j.jpeds.2008.05.005
12. Pereira JS, Forte GC, Drehmer M, Simon MI, Behling EB. Perfil nutricional de paciente com Fibrose Cística em Centro de Referência no Sul do Brasil. Rev HCPA. 2011;31(2):131-7.
13. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organization. 2007;85:660-7. http://dx.doi.org/10.2471/BLT.07.043497
14. Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34(11):2540-5.
15. Sociedade Brasileira de Pneumologia e Tisiologia. Diretrizes para testes de função pulmonar. J Pneumol. 2002;28(Suppl 3):S1-S238.
16. Shwachman H, Kulczycki LL. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J Dis Child. 1958;96(1):6-15.
17. Cystic Fibrosis Foundation. Patient Registry Annual Data Report 2009. Bethesda: Cystic Fibrosis Foundation; 2009.
18. Chaves CR, Britto JA, Oliveira CQ, Gomes MM, Cunha AL. Association between nutritional status measurements and pulmonary function in children and adolescents with cystic fibrosis. J Bras Pneumol. 2009;35(5):409-14.
19. Simon MI, Drehmer M, Menna-Barreto SS. Association between nutritional status and dietary intake in patients with cystic fibrosis. J Bras Pneumol. 2009;35(10):966-72.
20. Schall JI, Bentley T, Stallings VA. Meal patterns, dietary fat intake and pancreatic enzyme use in preadolescent children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2006;43(5):651-9. http://dx.doi.org/10.1097/01.mpg.0000234082.47884.d9
21. Döring G, Hoiby N; Consensus Study Group. Early intervention and prevention of lung disease in cystic fibrosis: a European consensus. J Cyst Fibros. 2004;3(2):67 91. http://dx.doi.org/10.1016/j.jcf.2004.03.008
22. Winklhofer-Roob BM. Cystic fibrosis: nutritional status and micronutrients. Curr Opin Clin Nutr Metab Care. 2000;3(4):293-7. http://dx.doi.org/10.1097/00075197-200007000-00009
23. Cantin AM. Bafilomycin A1, an inhibitor of vascular proton ATPase, suppresses glutathione synthesis in lung epithelial cells. Pediatr Pulmonol. 1999;19(Suppl):A307.
24. Abman SH, Reardon MC, Accurso FJ, Hammond KB, Sokol RJ. Hypoalbuminemia at diagnosis as a marker for severe respiratory course in infants with cystic fibrosis identified by newborn screening. J Pediatr. 1985;107(6):933-5. http://dx.doi.org/10.1016/S0022-3476(85)80194-3
25. Aurora P, Wade A, Whitmore P, Whitehead B. A model for predicting life expectancy of children with cystic fibrosis. Eur Respir J. 2000;16(6):1056-60. http://dx.doi.org/10.1034/j.1399-3003.2000.16f06.x
26. Pedreira CC, Robert RG, Dalton V, Oliver MR, Carlin JB, Robinson P, et al. Association of body composition and lung function in children with cystic fibrosis. Pediatr Pulmonol. 2005;39(3):276-80. http://dx.doi.org/10.1002/ppul.20162
27. Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303(23):2386-92.
Study carried out in the Nutrition and Dietary Department, Hospital de Clínicas de Porto Alegre, and at the Center for Food and Nutrition Studies, Porto Alegre, Brazil.
Correspondence to: Miriam Isabel Souza dos Santos Simon. Rua Ramiro Barcelos, 2350, CEP 90035-903, Porto Alegre, RS, Brasil.
Tel. 55 51 3359-8410. E-mail: misantos@hcpa.ufrgs.br
Financial support: This study received financial support from the Fundo de Incentivo à Pesquisa (FIPE, Research Incentive Fund) of the Hospital de Clínicas de Porto Alegre.
Submitted: 26 March 2012. Accepted, after review: 4 June 2012.
About the authorsGabriele Carra Forte
Graduate Student. Graduate Program in Pulmonology, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Juliane Silva Pereira
Nutritionist. Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Michele Drehmer
Adjunct Professor. Department of Social Medicine, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Miriam Isabel Souza dos Santos Simon
Nutritionist. Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil.




