ABSTRACT
Objective: To evaluate the influence of the surgical team (general surgery or thoracic surgery) and the surgical technique (with or without isthmectomy) on the incidence of postintubation injuries in the airways of tracheostomized patients. Methods: Between January 1st and August 31st, 2007, 164 patients admitted to the adult intensive care unit and tracheally intubated for more than 24 h were studied prospectively at the Sumaré State Hospital, located in the city of Sumaré, Brazil. When tracheostomy was necessary, these patients were randomly assigned to thoracic or general surgery teams. All of the patients were submitted to fiberoptic tracheoscopy for decannulation or late evaluation of the airway. Results: Of the 164 patients in the study, 90 (54.88%) died (due to causes unrelated to the procedure), 67 (40.85%) completed follow-up, and 7 (4.27%) were lost to follow-up. Of the 67 patients who completed follow-up, 32 had undergone tracheostomy (21 by the general surgery team and 11 by the thoracic surgery team), and 22 had been submitted to isthmectomy (11 by the general surgery team and 11 by the thoracic surgery team). There was no difference between the surgical teams in terms of the incidence of stoma complications. However, there was a significant difference when the surgical techniques (with or without isthmectomy) were compared. Conclusions: Not performing isthmectomy in parallel with tracheostomy leads the surgeon to open the tracheal stoma more distally than expected. In such cases, there were more stoma complications.
Keywords:
Tracheostomy; Intensive care units; Tracheal stenosis.
RESUMO
Objetivo: Avaliar a influência da equipe cirúrgica (cirurgia geral ou cirurgia torácica) e da técnica operatória utilizada (com ou sem istmectomia) sobre a incidência de injúrias pós-intubação nas vias aéreas em pacientes traqueostomizados. Métodos: Foram prospectivamente incluídos neste estudo 164 pacientes admitidos na unidade de terapia intensiva para adultos do Hospital Estadual Sumaré e que ficaram sob intubação traqueal por mais de 24 h, no período entre 1º de janeiro e 31 de agosto de 2007. Quando foi necessária a realização de traqueostomia, os pacientes foram aleatorizados para as equipes de cirurgia geral e torácica. Todos os pacientes foram submetidos à traqueoscopia flexível para a decanulação e/ou a avaliação tardia da via aérea. Resultados: Dos 164 pacientes no estudo, 90 (54,88%) faleceram (sem relação com o procedimento), 67 (40,85%) completaram o seguimento e 7 (4,27%) tiveram seguimento parcial. Dos 67 pacientes com seguimento completo, 32 foram traqueostomizados (21 pela equipe de cirurgia geral e 11 pela equipe de cirurgia torácica). A istmectomia foi realizada em 22 pacientes (11 pela equipe de cirurgia geral e 11 pela equipe de cirurgia torácica). Não houve diferença entre o índice de complicações estomais quando se comparou as equipes, mas sim quando se comparou as técnicas (com e sem istmectomia). Conclusões: A não realização da istmectomia paralelamente à traqueostomia faz com que o cirurgião realize o óstio traqueal mais distalmente do que supõe. Nestes casos, houve um maior índice de complicações do estoma traqueal.
Palavras-chave:
Traqueostomia; Unidades de terapia intensiva; Estenose traqueal.
IntroductionOpen surgical tracheostomy continues to be, in most health care facilities, the most widely used procedure for the prevention of laryngeal complications as a consequence of prolonged intubation,(1,2) despite the increasing popularity of minimally invasive techniques of percutaneous tracheostomy.(3,4)
It is known, however, that an inappropriate surgical technique may lead to alterations in the tracheal stoma.(1,2,5) Therefore, elective surgery aimed at preventing a laryngeal complication leads to a stoma complication that would not otherwise exist.
There is no consensus among general surgeons and specialists in respiratory medicine regarding the ideal surgical technique, the choice of surgical technique varying based on the professional experience of each surgeon.
Risk factors for the development of postintubation lesions in the airways include the following: infection; high pressure in the cuff and in the tube itself; hemodynamic shock; and the technique used.(5-7) A number of technical details of open surgical tracheostomy, such as the cannula number used in relation to the size of the tracheal opening, the tracheal ring used for the conduction of the stoma and the performance (or not) of isthmectomy, have no well-established standardization and some textbooks have no defined position regarding the need for these procedures.(8,9)
This cohort study aimed to evaluate the influence of the surgical team (general surgery or thoracic surgery) and the surgical technique (with or without isthmectomy) used on the incidence of postintubation injuries in the tracheal stoma and in the airways of tracheostomized patients.
MethodsAll patients admitted to the Adult Intensive Care Unit (ICU) of the Sumaré State Hospital, State University at Campinas, between January 1st and August 31st, 2007 and remaining under tracheal translaryngeal intubation (orotracheal or nasotracheal) for more than 24 h were included in this prospective study.
Patients who had come from other facilities were excluded, as were those already under mechanical ventilation, those submitted to other surgical procedures involving the airway, those that had been intubated or tracheostomized in the six months prior to the study and those whose family members did consent to their participation in the study.
For comparison between the populations, demographic data (gender, age and ethnicity) were collected, as were scores, such as the Acute Physiology and Chronic Health Evaluation (APACHE), as well as the reason that led the patient to intubation.
The ICU team was responsible for making the decision as to length of time on translaryngeal intubation after which tracheostomy was indicated. After the decision regarding tracheostomy had been made, the patients were randomized (through a process of drawing opaque sealed envelopes) to undergo the procedure performed either by the general surgery team or by the thoracic surgery team.
The general surgery team was composed of six gastrointestinal surgeons, all with experience in performing tracheostomies, whereas a single surgeon was responsible for all of the tracheostomies performed by the thoracic surgery team.
The choice of surgical technique (with or without isthmectomy) was the responsibility of the surgeon in charge, as was choice of the tracheal ring in which the tracheal stoma would be created and the size of the cannula to be used. Immediately following the procedure, the surgeon in charge was interviewed regarding these data. The tracheal ring in which, according to the surgeon, the stoma had been created was designated the supposed tracheal ring. When isthmectomy was not performed, the isthmus was folded upward (cranially).
As soon as the tracheostomized patient was weaned from mechanical ventilation, the plastic cuffed tracheostomy cannula was replaced with a metallic cannula.
After hospital discharge, all patients were referred to the outpatient thoracic surgery clinic for clinical and endoscopic evaluation. Tracheostomized patients were also submitted to decannulation.
At 60 days after undergoing tracheostomy, the patients were submitted to fiberoptic tracheoscopy under local anesthesia at an outpatient clinic. The tracheal ring into which the cannula was inserted was then designated the true tracheal ring. Alterations in the tracheal stoma, such as granulomas, stenosis and tracheomalacia were evaluated, as well as other changes in the airways (laryngeal, suprastomal and infrastomal alterations). The endoscopist was blinded to the technique employed and to the team conducting the procedure.
For the final evaluation and for analysis of the criteria for discharge from the outpatient clinic, all patients, whether tracheostomized or not, were again submitted to fiberoptic laryngotracheoscopy at 180 days after the surgical procedure. When symptoms of respiratory failure occurred, the patient was submitted to emergency bronchoscopy (with a fiberoptic or rigid bronchoscope). Therefore, the clinical and endoscopic follow-up period for the diagnosis and treatment of possible sequelae in the airways was 180 days for all patients.
For the statistical analysis, we used the program Number Cruncher Statistical System (NCSS Inc., Kaysville, UT, USA); unpaired Student's t-test was used for parametric data, and Fisher's exact test or chi-square test was used for ordinal or nominal data, with a level of statistical significance of 0.05 for differences between the populations.
This study was approved by the Ethics in Research Committee of the State University at Campinas School of Medical Sciences under ruling no. 528/2007. All participating patients, or their families, gave written informed consent.
ResultsA total of 164 consecutive patients were studied prospectively between January 1st and August 31st, 2007.
Of these patients, 67 (40.85%) completed the proposed minimum clinical follow-up period of 60 days, 90 (54.88%) died during hospitalization or prior to the first endoscopic evaluation (at 60 days), and 7 (4.27%) were lost to follow-up. The APACHE score of those who completed the follow-up period was 19.41 ± 7.53, whereas, in those who died, it was 29.95 ± 10.16, with a significant difference between the two populations (p < 0.001). There were no differences in demographic data or in the reasons for intubation.
Of the 164 patients, 55 (33.53%) were submitted to tracheostomy. The thoracic surgery team performed 23 (41.82%) of the 55 procedures. Of the 55 patients submitted to tracheostomy, 32 (58.18%) survived.
We assumed, as a final sample, 67 patients (those who completed the follow-up period and in whom it was consequently possible to evaluate outcomes). Of these 67 patients, 39 (58.21%) were males. Ages ranged from 15 to 77 years, with a mean of 45.55 ± 19.55 years. Of the 67 patients, 57 (85.07%) were white, 7 (10.45%) were black, and 3 (4.48%) were mulatto.
As shown in Figure 1, the reasons for intubation and prolonged ventilatory support were the following: postoperative support, in 18 patients (26.87%); severe craniocerebral trauma, in 14 (20.90%); and respiratory infections, in 9 (13.43%).
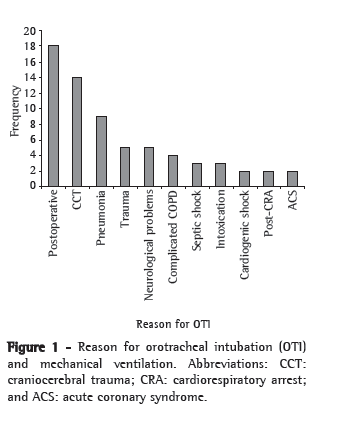
Of the 67 patients, 32 (47.76%) were submitted to open tracheostomy by translaryngeal intubation, which was orotracheal in all cases. The mean duration of translaryngeal intubation in non-tracheostomized patients was 5.54 ± 3.63 days (range, 1-14 days), compared with 10.71 ± 2.50 days (range, 6-17 days) in tracheostomized patients.
Among the 32 survivors, the tracheostomy had been performed by the general surgery team in 21 cases (65.63%) and by the thoracic surgery team in 11 (34.37%). All procedures conducted by the thoracic surgery team included isthmectomy, which, in contrast, was performed in only 11 (52.38%) of the 21 procedures conducted by the general surgery team (p = 0.0057).
In relation to the supposed tracheal ring, the general surgery team stated that the stoma was created in the second and third tracheal rings in 17 and 4 patients, respectively. According to the thoracic surgery team, the stoma was created in the second tracheal ring in all of their patients.
At the time of decannulation (true tracheal ring), the actual location of the stomas in the patients submitted to the procedure by the general surgery team was in the second tracheal ring in 12 patients, the third tracheal ring in 5, the fourth tracheal ring in 2, the fifth tracheal ring in 1 and the sixth tracheal ring in 1. In the patients submitted to the procedure by the thoracic surgery team, all stomas were found to be in the second tracheal ring. The supposed and true tracheal ring were different in 9 (42.86%) of the 21 cases in which no isthmectomy was performed (p = 0.019). Among the 11 cases in which the procedure was conducted by the general surgery team and included isthmectomy, there was, at the time of decannulation, a difference between the supposed and true tracheal ring in 3 cases, compared with 6 of the 10 cases in which isthmectomy was not performed (p = 0.198; Figure 2).
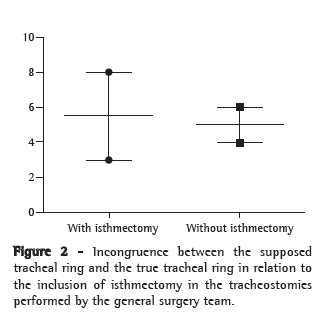
Among the 32 tracheostomies performed, there were stoma complications (granulomas, tracheomalacia or stenosis) in 13 (40.62%). When the two teams were compared, complications were found in 11 general surgery group patients and 2 thoracic surgery group patients (p = 0.065). There were no differences when the stoma complications were compared in relation to whether or not isthmectomy was performed (p = 0.132), regarding the congruence between the supposed tracheal ring and the true tracheal ring (p = 0.070), as well as the size of the tracheostomy cannula used (p = 0.385). When the true tracheal ring was compared with stoma complications at the time of decannulation, no differences were found between the populations (p = 0.144). However, stoma complications occurred in all 4 of the patients in which the stoma was located below the third tracheal ring, compared with only 9 (47.37%) of the 19 patients in which the stoma was located in the second or third tracheal rings (p = 0.01). The determining factor for the creation of the stoma below the third tracheal ring, present in 3 of the 4 cases, was not performing isthmectomy (p = 0.02). There was no difference between the patients with stoma complications and those without in terms of the duration of translaryngeal intubation preceding the tracheostomy (p = 0.891).
Summing the data collected on postoperative day 60 (Table 1) and on postoperative day 180 (Table 2), a total of 2 patients (3%) presented supraglottic complications (after two and four days of translaryngeal intubation, respectively), 6 (9%) presented subglottic complications, 13 (19.4%) presented stoma complications, and 3 (4.5%) presented infrastomal or tracheal complications. Of the 32 tracheostomized patients, 14 (43.8%) presented normal laryngotracheoscopy findings on postoperative day 60 (Table 1), compared with 55 (82.1%) on postoperative day 180 (Table 2).
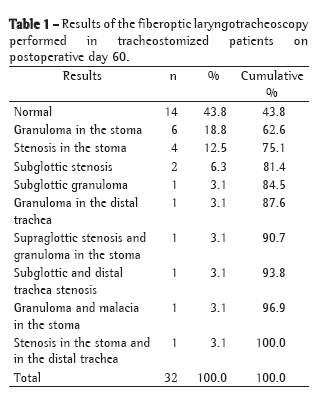
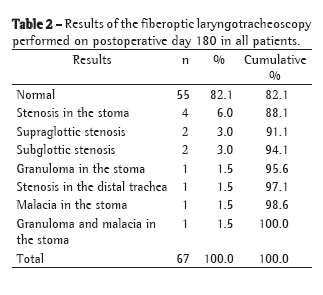
Intervention was necessary for the treatment of sequelae in 5 (7.46%) of the 67 patients evaluated: 1 patient was submitted to a resection with end-to-end anastomosis of the trachea; decannulation was impossible in 1 (in whom surgery was indicated but not recommended); and 3 received Montgomery T-tube type tracheal stents. All reconstructive interventions in the airway were performed in tracheostomized patients. Of these 5 patients, 3 (those with tracheal resection, impossibility of decannulation and placement of Montgomery T-tube) had shown alterations in the tracheal stoma, all created below the third tracheal ring; the other 2 received Montgomery T-tubes due to stenosis of the distal trachea.
DiscussionCervical tracheostomy is an old procedure,(10,11) which has been described thousands of years ago and is the most common surgical procedure performed in critically ill patients.(11) Use of the procedure expanded after an outbreak of poliomyelitis, and complications began to appear in the 1960s(1,6) following the advent of tracheostomy tubes with high pressure cuffs. Subsequently, despite the use of tubes with low pressure cuffs (translaryngeal or tracheostomy), a wide range of secondary benign injuries in the airways have been treated.(12) At most health care facilities, tracheostomy is considered a preventive and elective procedure aimed at minimizing laryngeal sequelae due to prolonged translaryngeal tracheal intubation, as well as having been shown to be related to favorable early in-hospital evolution.(13-15)
The principal motivation for the performance of tracheostomy is prolonged translaryngeal intubation,(15) which, at most health care facilities, is used for a maximum of 10 to 12 days, as recommended.(16) Other motivations include the following: facilitating tracheobronchial hygiene and management; making the patient comfortable; ensuring that the nursing and physiotherapy teams can work more safely; and stabilizing the airway. However, these recommendations are rarely measured, and the level of technical acceptance is therefore low.(15)
Therefore, open or minimally invasive cervical tracheostomy, when recommended due to prolonged translaryngeal intubation, has a preventive and elective aspect. The surgical technique has to be applied with precision for a procedure with these characteristics not to become a new source of acute and chronic complications, with stoma alterations (granulomas, tracheomalacia and stenosis) which prevent or preclude decannulation, causing invasive endoscopic or surgical corrective procedures in patients already debilitated by their stay in the ICU.
It should not be delegated to less experienced surgeons or to residents alone, and it should always be performed in the presence of an attending surgeon/supervisor (rather than being "phoned in" by the same)-not simply the most senior resident. Health care facilities should make every effort to improve the technique.
The following are determining factors for preventing complications in the tracheal stoma: an ample stoma, with no cartilage resection; an aseptic technique; and the use of a cannula of the appropriate size. Isthmectomy seems to play a secondary role in the opinion of the surgeons; however, it is impossible to be sure about the exact placement of the tracheal stoma if isthmectomy is not performed, as shown in this clinical study.
In the present study, we found that, when the tracheostomy did not include isthmectomy, surgeons created the stoma in tracheal rings that were more distal than expected, and there was a correlation between creating the stoma below the third tracheal ring and the occurrence of stoma complications.
There was no statistical difference between the procedures performed by the general surgery team and those performed by the thoracic surgery team in terms of the frequency of stoma complications, although there was a tendency toward significance (p = 0.065), as there was between the teams for the congruence of the supposed tracheal ring with the true tracheal ring (p = 0.070). This may be due to the fact that the sample, despite being consecutive,(17) when divided in groups and including only the survivors, was significantly decreased. However, we should consider the fact that the thoracic surgery team conducted exclusively open tracheostomy with isthmectomy and therefore always created the stoma in the second tracheal ring, which resulted in fewer complications than those seen after the procedures performed by the general surgery team, who did not always perform isthmectomy, leading to considerable incongruence between the supposed and the true tracheal ring, which presented a direct correlation with stoma complications, therefore requiring that additional surgical and endoscopic procedures be performed.
It is of note that, in 3 of the cases in which the general surgery team also performed isthmectomy, the true tracheal ring differed from the supposed tracheal ring, indicating that, even when isthmectomy is performed, special attention should be given to the correct identification of the cricoid cartilage and tracheal rings.
We opted for decannulation after 60 days, a conservative approach taken in order to safely evaluate and treat of negative outcomes, which constituted the aim of our study. This decision was based on previous data demonstrating that this is the critical period for the appearance of injuries secondary to intubation.(1,5) Tracheoscopy performed at 60 days for decannulation was the key point of the present study. However, in our health care facility, this is a common procedure for tracheal decannulation, since the occlusion of the cannula test only detects 50% of airway injuries.(5,18) In addition, subcritical stenosis can go undiagnosed and dyspnea can be attributed to other factors, such as recent pulmonary injury, loss of muscle strength and protein-calorie malnutrition, limiting the possibility of early elective treatment(19) and promoting a less favorable evolution.
Although it was not our objective to study the level at which tracheostomies are performed, we found that creating the stoma in a lower tracheal ring was the principal risk factor for a serious and typically fatal complication, namely tracheo-innominate artery fistula, which is caused by erosion of the wall by the cannula or cuff, bringing the trachea and the brachiocephalic artery into opposition.(5,20) It is known that postintubation stenosis occurs more frequently in subglottic, suprastomal and stomal sites.(1) In candidates for surgical treatment involving resection of the stenosis or end-to-end anastomosis of the airway, low placement of the stoma, distant from the suprastomal stenosis, can make it difficult or even impossible to perform the corrective surgery.(21) In our sample, only 1 patient was a candidate for tracheal resection involving end-to-end anastomosis and 3 required tracheal stents.(22,23)
Despite the increasing interest in and the applicability of tracheostomy said to be minimally invasive with percutaneous techniques,(24) tracheoscopy-guided or otherwise, open tracheostomy remains the technique used in most public hospitals, and every effort should be made to avoid further injury in patients already debilitated and traumatized by long, punishing hospital stays.
Recently, factors not measured clinically and which are not part of the routine of the ICU and specialized outpatient clinics have been related to complications in the airways.(25) This can effectively be a promising line of research in the prevention of these diseases. However, we must currently focus on measurable day-to-day aspects as simple as choosing the appropriate surgical procedure.
In conclusion, we are in favor of performing isthmectomy in order to ensure that the stoma is created in the second or third tracheal ring. We believe that the following factors are important: the creation of an ample stoma, without resection of the cartilaginous tissue; avoiding inversion of the edges of the stoma in relation to tracheal lumen; careful selection of a cannula that is of an appropriate size (compatible with the size of the trachea), thereby avoiding excessive pressure on the tracheal wall and the need for high cuff pressure in order to fully occlude the airway; additional precautions, so this preventive, elective procedure does not become a source of complication in critically ill patients, such complications being related to the technique employed rather than to the makeup of the team that conducts the procedure.
References 1. Maddaus MA, Pearson FG. Postintubation injury. In: Pearson FG, Patterson GA, editors. Pearson's Thoracic and Esophageal Surgery. Philadelphia: Churchill Livingstone/Elsevier; 2002. p. 300-314.
2. Streitz JM Jr, Shapshay SM. Airway injury after tracheotomy and endotracheal intubation. Surg Clin North Am. 1991;71(6):1211-30.
3. Park M, Brauer L, Sanga RR, Kajdacsy-Balla AC, Ladeira JP, Azevedo LC, et al. Percutaneous Tracheostomy in Critically-ill Patients: The Experience of a Medical Intensive Care Unit. J Bras Pneumol. 2004;30(3):237-242.
4. Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy. A new simple bedside procedure; preliminary report. Chest. 1985;87(6):715-9.
5. Epstein SK. Late complications of tracheostomy. Respir Care. 2005;50(4):542-9.
6. Pearson FG, Goldberg M, da Silva AJ. A prospective study of tracheal injury complicating tracheostomy with a cuffed tube. Ann Otol Rhinol Laryngol. 1968;77(5):867-82.
7. Braz JR, Navarro LH, Takata IH, Nascimento Júnior P. Endotracheal tube cuff pressure: need for precise measurement. Sao Paulo Med J. 1999;117(6):243-7.
8. Goldstraw P, Morgam C. Tracheostomy. In: Pearson FG, Patterson GA, editors. Pearson's Thoracic and Esophageal Surgery. Philadelphia: Churchill Livingstone/Elsevier; 2002. p. 375-383.
9. Putnam Jr JB. Traquéia. In: Townsend MC, editor. Sabiston Tratado De Cirurgia. Rio de Janeiro: Elsevier; 2005. p. 1792-1793.
10. Vianna A. Tracheostomy in patients on mechanical ventilation: when is it indicated? J Bras Pneumol. 2007;33(6):xxxvii-xxxviii.
11. Perfeiro JA, Mata CA, Forte V, Carnaghi M, Tamura N, Leão LE. Tracheostomy in the ICU: is it worthwhile? J Bras Pneumol. 2007;33(6):687-90.
12. Leite AG, Kussler D. Management of recurrent distal tracheal stenosis using an endoprosthesis: a case report. J Bras Pneumol. 2008;34(2):121-5.
13. Arabi Y, Haddad S, Shirawi N, Al Shimemeri A. Early tracheostomy in intensive care trauma patients improves resource utilization: a cohort study and literature review. Crit Care. 2004;8(5):R347-52.
14. Croshaw R, McIntyre B, Fann S, Nottingham J, Bynoe R. Tracheostomy: timing revisited. Curr Surg. 2004;61(1):42-8.
15. Walts PA, Murthy SC, Arroliga AC, Yared JP, Rajeswaran J, Rice TW, et al. Tracheostomy after cardiovascular surgery: an assessment of long-term outcome. J Thorac Cardiovasc Surg. 2006;131(4):830-7.
16. Plummer AL, Gracey DR. Consensus conference on artificial airways in patients receiving mechanical ventilation. Chest. 1989;96(1):178-80.
17. Leung R, MacGregor L, Campbell D, Berkowitz RG. Decannulation and survival following tracheostomy in an intensive care unit. Ann Otol Rhinol Laryngol. 2003;112(10):853-8.
18. Pinet C, Quenee V, Sainty JM. Significance of systematic endoscopic decannulation. Retrospective study on intensive care patients [Article in French]. Rev Pneumol Clin. 1998;54(2):81-4.
19. Nouraei SA, Singh A, Patel A, Ferguson C, Howard DJ, Sandhu GS. Early endoscopic treatment of acute inflammatory airway lesions improves the outcome of postintubation airway stenosis. Laryngoscope. 2006;116(8):1417-21.
20. Coelho MS, Zampier JA, Zanin SA, Silva EM, Guimarães PS. Fístula traqueoesofágica como complicação tardia de traqueostomia. J Pneumol. 2001;27(2):119-22.
21. Grillo HC, Mathisen DJ, Wain JC. Laryngotracheal resection and reconstruction for subglottic stenosis. Ann Thorac Surg. 1992;53(1):54-63.
22. Saueressig MG, Macedo-Neto AV, Moreschi AH, Xavier RG, Sanches PR. A correção das estenoses traqueobrônquicas mediante o emprego de órteses. J Pneumol. 2002;28(2):84-93.
23. Terra RM, Minamoto H, Tedde ML, Almeida JL, Jatene FB. Self-expanding stent made of polyester mesh with silicon coating (Polyflex®) in the treatment of inoperable tracheal stenoses. J Bras Pneumol. 2007;33(3):241-7.
24. Gravvanis AI, Tsoutsos DA, Iconomou TG, Papadopoulos SG. Percutaneous versus Conventional Tracheostomy in Burned Patients with Inhalation Injury. World J Surg. 2005;29(12):1571-5.
25. Karagiannidis C, Velehorschi V, Obertrifter B, Macha HN, Linder A, Freitag L. High-level expression of matrix-associated transforming growth factor-beta1 in benign airway stenosis. Chest. 2006;129(5):1298-304.
Study carried out in the Thoracic Surgery and Respiratory Endoscopy Department and in the Adult Intensive Care Unit of the Sumaré State Hospital, Universidade Estadual de Campinas - Unicamp, State University at Campinas - Campinas, Brazil.
Correspondence to: Alexandre Garcia de Lima. Rua João Simões da Fonseca, 70, Condomínio Residencial Barão do Café 4, CEP 13085-050, Campinas, SP, Brasil.
Tel 55 19 2117-3300. E-mail: alexandre.garcia@toracica.com.br
Financial Support: None.
About the authorsAlexandre Garcia de Lima
Head of the Thoracic Surgery and Respiratory Endoscopy Department of the Sumaré State Hospital, Universidade Estadual de Campinas - Unicamp, State University at Campinas - Campinas, Brazil.
Ariovaldo Marques
Head of the Adult Intensive Care Unit of the Sumaré State Hospital, Universidade Estadual de Campinas - Unicamp, State University at Campinas - Campinas, Brazil.
Ivan Felizardo Contrera Toro
Head of Thoracic Surgery at the Universidade Estadual de Campinas - Unicamp, State University of Campinas - Hospital das Clínicas, Campinas, Brazil.





