ABSTRACT
Objective: To determine the relationship between nutritional status and dietary intake in patients with cystic fibrosis. Methods: Cross-sectional study involving 85 cystic fibrosis patients between 6 and 18 years of age. Dietary intake was evaluated by the 3-day diet record (weighing the food consumed). The outcome measures were the following nutritional status indicators: weight/height (W/H%) percentage, body mass index (BMI) percentiles, Z score for weight/age (W/A), Z score for height/age (H/A) and percentage of dietary intake compared with the Recommended Dietary Allowance (RDA). Results: The prevalence of well-nourished patients was 77.7%, using BMI above the 25th percentile as the cut-off value, and the W/H% was above 90% in 83.5%. The mean dietary intake, evaluated in 82 patients, was 124.5% of the RDA. In the univariate logistic regression analyses, we found a significant association between the independent variable calorie intake and the Z score for W/A. The multivariate analysis, based on the Z score for H/A and adjusted for FEV1, methicillin-resistant Staphylococcus aureus colonization and number of hospitalizations, demonstrated that a 1% increase in the calorie intake decreases the chance of having short stature by 2% (OR: 0.98; 95% CI: 0.96-1.00). Maternal level of education showed a borderline association (p = 0.054). Conclusions: The prevalence of malnutrition was low in this sample of patients. The study model demonstrated an association between dietary intake and nutritional status. Dietary intake was a predictive factor of statural growth in patients with cystic fibrosis.
Keywords:
Cystic fibrosis; Nutritional status; Diet records; Child; Adolescent.
RESUMO
Objetivo: Determinar a relação entre o estado nutricional e a ingestão dietética de pacientes com fibrose cística. Métodos: Estudo transversal com 85 pacientes com fibrose cística entre 6 e 18 anos de idade. A ingestão dietética foi avaliada pelo registro alimentar de 3 dias com a pesagem dos alimentos consumidos. Os desfechos avaliados foram os seguintes indicadores do estado nutricional: percentual da relação peso/estatura (%P/E), percentil do índice de massa corpórea (IMC), escore Z para estatura/idade (E/I) e peso/idade (P/I), e percentual de ingestão dietética comparada a Recommended Dietary Allowances (RDAs). Resultados: A prevalência de pacientes eutróficos foi de 77,7%, considerando o IMC acima do percentil 25 como ponto de corte, e 83,5% estavam acima de 90% do %P/E. A média de ingestão, avaliada em 82 pacientes, foi de 124,5% da RDA. Nas análises de regressão logística univariada, encontrou-se uma associação significativa entre a variável independente ingestão calórica e o desfecho escore Z E/I. O modelo de análise multivariado, elaborado a partir do desfecho escore Z E/I e ajustado para idade, VEF1, colonização por Staphylococcus aureus resistente à meticilina e número de internações hospitalares, demonstrou que um aumento de 1% da ingestão calórica em relação à RDA diminui em 2% a chance de ter déficit de estatura (OR = 0,98; IC95%: 0,96-1,00). A escolaridade materna demonstrou uma associação limítrofe (p = 0,054). Conclusões: Houve baixa prevalência de desnutrição nesta amostra. O modelo de estudo demonstrou evidências da associação entre a ingestão dietética e o estado nutricional, sendo esta ingestão um fator preditor de crescimento nesses pacientes.
Palavras-chave:
Fibrose cística; Estado nutricional; Registros de dieta; Criança; Adolescente.
IntroductionMalnutrition is a leading cause of mortality in children, youths and adults with cystic fibrosis (CF) and is associated with the progression of the disease. The factors that contribute to the perpetuation of the impaired nutrition status can be related to the increase in nutritional need and the decrease in food intake, as well as to greater dietary deficiencies.(1)
The increase in the energy requirements, the decrease in intake and the greater dietary deficiencies are related to the deterioration of pulmonary function, anorexia, vomiting, pancreatic insufficiency, chronic inflammatory activity, biliary complications and intestinal complications, resulting in a loss of lean body mass and impaired immune function.(2)
The recommendation of daily energy intake is based on the elevation of energy expenditure presented by these patients. It is known that CF patients require at least 120-150% of the energy intake established in the Recommended Dietary Allowances (RDAs) of 1989.(3) There is no indication of the percentage of that greater need, since it depends on the degree of intestinal absorption and the metabolic rate.(2)
Despite the advances in clinical and nutritional treatment, the Cystic Fibrosis Foundation, which compiles the data of American CF patients, reported that 15.7% and 16.3% of these patients presented values lower than the 5th percentile for weight and height, respectively, in 2004.(4) In 1993, the Latin-American Cystic Fibrosis Registry demonstrated that over 50% of the patients were below the 3rd percentile for weight and that 46.7% of the patients were below the same percentile in relation to height for age.(5)
The pattern of dietary intake in CF patients seems to be directly related to their growth and to nutritional status. However, height can also be related to survival in these patients.(6) This study aimed to determine the relationship between nutritional status and dietary intake of CF patients aged 6-18 years and treated at a referral center in Brazil.
MethodsThis was a cross-sectional study. Data were collected between July of 2004 and December of 2005, during routine CF outpatient follow-up. The following patients were consecutively included: all CF patients (n = 88) aged between 6 and 18 years, not colonized by Burkholderia cepacia and under outpatient treatment by the Pediatric Pulmonology team of the Hospital de Clínicas de Porto Alegre (HCPA, Porto Alegre Hospital de Clínicas), located in the city of Porto Alegre, Brazil. The inclusion criteria were as follows: having a clinical history of CF; having high levels of sodium and chloride in sweat; or the identification of two mutations. We excluded 2 patients presenting terminal disease and 1 patient due to the impossibility of obtaining written informed consent.
A chart with the following fields was used as a tool for data collection: date of birth; gender; maternal level of education; enzyme and vitamin supplementation; bacterial colonization; FEV1; and the number of hospitalizations since diagnosis. Pancreatic insufficiency was determined by evaluation of the use of pancreatic enzymes. Bacterial colonization was determined based on the evaluation of sputum samples collected during the preceding twelve months.
Spirometry was carried out in order to measure FEV1, the parameter most commonly used to quantify the obstructive respiratory defect typical in CF. Flow-volume curves were carried out with a Master Screen spirometer (Jaeger, Würzburg, Germany), using the Zapletal et al. table for the predicted values.(7) Spirometry was always conducted by the same rater, and the quality was evaluated by the attending physician based on the analysis of the curves.
Dietary intake was evaluated with a food registry maintained over three nonconsecutive days, which involved the weighing of the foods consumed. A scale with maximum capacity of 2 kg and minimum gradations of 25 g was provided for food weighing. In order to measure liquids, a plastic beaker with a capacity of 600 mL and minimum gradations of 10 mL was provided. The patient or legal guardian was trained to correctly fill out the three-day food registry.(8) The survey was conducted referring to two nonconsecutive week days and one weekend day, and all the home preparations were registered, with the proper ingredients used, in addition to the quantity of oil used by the family per month. The calculation of the food registry was carried out using the software Decision in Nutrition Support System of the Paulista School of Medicine(9) and by comparison with the RDAs.(3)
The anthropometric evaluation took place during the medical visit, after written informed consent had been given by the parents or legal guardians. Weight and height were always measured by the same rater, who used standardized techniques. Weight was obtained with the patient wearing only a standard hospital gown, with an electronic scale (Filizola, São Paulo, Brazil) with maximum load of 150 kg and variation of 50 g. Height was measured using a Sanny stadiometer (American Medical do Brasil, São Bernardo do Campo, Brazil) affixed to a wall. Patients wore no shoes or hair accessories and stood with their heels together, back against the anthropometer, arms relaxed at their sides and head in the vertical position, looking straight ahead. While the evaluated patient inhaled, the horizontal bar was lowered to the highest point of the head. Arm circumference (AC) was measured at the midpoint of the non-dominant arm using a flexible non-elastic tape measure. Triceps skinfold thickness (TST) was measured at the midpoint of the non-dominant arm using a skinfold caliper (Harpenden; British Indicators, Burguess Hill, UK). Mid-arm muscle circumference (MAMC) was calculated using the following equation:
MAMC (cm) = AC − TST (mm) × 0.314. All values were compared with the criteria established by Frisancho.(10)
The nutritional parameters used were the Z score for weight and age (W/A) and height and age (H/A), in accordance with the National Center for Health Statistics, body mass index (BMI) percentiles and the weight/height percentage (W/H%), calculated by dividing the current weight and the percentile weight corresponding to the height × 100. These last two parameters were used because they are nutritional indicators recommended in two consensuses,(11,12) in which the cut-off values for nutritional failure, nutritional risk and malnutrition, respectively, were established at lower than 90% of W/H%, BMI between the 10th and 25th percentiles and BMI below the 10th percentile.
In the data analysis, frequencies, means and medians of the dependent and independent variables were calculated. Univariate logistic regression analysis was also used between the studied factors with outcomes that represented nutritional status (W/H%; Z score for H/A; Z score for W/A; and BMI percentile). Variables that presented p ≤ 0.25 were included in the multivariate model. The level of significance was set at 5%, and the analyses were carried out using the Statistical Package for the Social Sciences, version 12.0 (SPSS Inc., Chicago, IL, USA).
The study was approved by the Research Ethics Committee of the Postgraduate and Research Group of the HCPA (Project no. 03-389). The necessary resources for the purchase of scales and the plastic beakers were provided by the Research Incentive Fund.
ResultsThe sample comprised 85 patients, and 55.3% were male, with a mean age of 11.2 ± 3.2 years. Ages ranged from 6.0 to 17.5 years. Of those 85 patients, 39 (45.9%) were from Porto Alegre or its greater metropolitan region, 17 (20.0%) were from the northeast region of the state (Rio Grande do Sul), and the remaining patients were from other regions of the state. Pancreatic insufficiency was present in 87.1% of patients. Vitamin supplementation was used regularly by 97.6% of the patients. The nutritional supplements used regularly by these patients comprised glucose polymers and a hypercaloric liquid diet (1.5 kcal/mL); 65.9% of the patients used one or more oral supplements, and 9.4% were receiving nocturnal supplementation through gastrostomy.
The mean number of years of maternal education was 9.0 ± 3.8, and 34% of the mothers presented less than 9 years of schooling. As for the presence of bacterial colonization in the preceding year, 74.1% of the patients presented Staphylococcus aureus, 18.8% of those presented methicillin-resistant S. aureus (MRSA), 52.9% of those presented Pseudomonas aeruginosa, and 24.7% of those presented mucoid P. aeruginosa. The mean FEV1 was 84.1 ± 24.4%. The median number of hospitalizations was 5, with an interquartile range from 2 to 11.
According to the four indicators of nutritional status evaluated in our study, all means were within the normal nutritional status range, demonstrating the appropriate nutritional status observed in our sample (Table 1). In our study, 16.5% of the patients presented a W/H% equal to or lower than 90%, 14.1% presented a BMI between the 10th and 25th percentiles, and 8.2% presented a BMI below the 10th percentile.
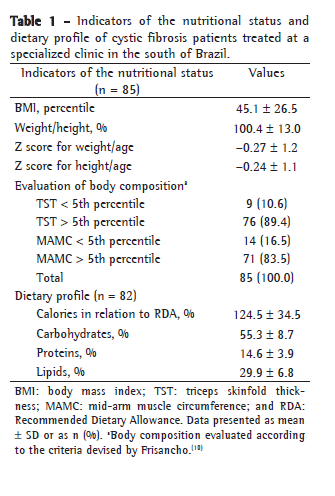
Measurement of the TST revealed that the fat reserve was depleted in 10.6% of the patients. There was depletion of muscle mass, as estimated by measurement of the MAMC, in 16.5% of the patients (Table 1).
The mean percentage of caloric intake, evaluated in 82 patients, was 124.6% of the RDA. The proportion of patients who presented an energy intake greater than 120% of the RDA was 51.7% (Table 1).
Figure 1 shows that the median BMI among patients who had a caloric intake greater than 120% of the RDA was at the 56th percentile, compared with approximately the 35th percentile for those who ingested less than the RDA of calories.
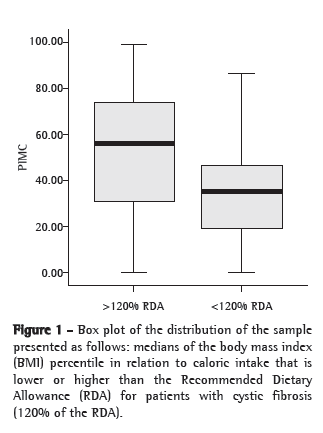
Table 2 presents the association between caloric intake and nutritional status, evaluated through the dependable variables W/H%, BMI percentile, Z score for H/A and Z score for W/A, based on the analysis of the univariate logistic regression. The indicators of nutritional status studied were dichotomized, using malnutrition cut-off points in accordance with the CF consensuses(11,12) and the World Health Organization.(13) In the crude analysis, there was a significant association between the independent variable caloric intake and the Z score for H/A outcome.
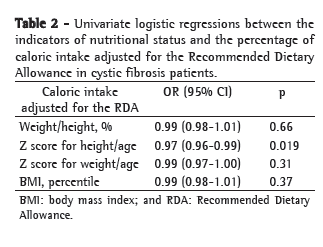
The multivariate analysis model was developed based on the Z score for H/A outcome, since an association between this outcome and caloric intake was observed in the crude analysis. Based on the hypothesis that caloric intake is associated with growth, independent variables which had p ≤ 0.25 in the crude analysis with the Z score for H/A were included.
Therefore, using the Z score for H/A dichotomized in normal nutritional status and abnormal nutritional status (greater than −1,28), we included the following variables in the model: caloric intake adjusted for the RDA; maternal level of education; colonization with MRSA; FEV1; age; and number of hospitalizations. This logistic regression model, in accordance with the Hosmer-Lemeshow goodness-of-fit test, showed the adjusted chi-square statistic to be 5.57 (p = 0.7).
As can be seen in Table 3, the 1% increase, in relation to the RDA, in caloric intake (when adjusted for age, FEV1, colonization with MRSA and number of hospitalizations), decreases the chance of height deficit by 2% (OR = 0.98; 95% CI: 0.96-1.00). Maternal level of education seems to influence the nutritional indicator related to growth, that is, having a mother who has had more years of schooling seems to have a protective effect on the nutritional indicator related to growth. However, in the multivariate analysis, this association remained only borderline significant (p = 0.054).
 Discussion
DiscussionThe nutritional parameters in this sample can be compared with those of the patients treated at CF centers in developed countries(4) due to the low prevalence of malnutrition, even if those parameters are evaluated using the strict criteria established by the CF consensuses and considering the socioeconomic conditions of a developing country. It is supposed that such findings can be related to the multidisciplinary management of a referral center, as observed in one study.(14) A study conducted in the United States and involving 22,714 CF patients showed a tendency toward improvement of the nutritional status with age in these patients in the last decade.(4)
Data from the United Kingdom Cystic Fibrosis Registry revealed that the mean Z score for the weight of boys up to 10 years of age was between −0.25 and −0.5; after that age bracket, there was a decrease in the BMI. Girls presented a Z score of −0.5; with a drop in the BMI after 5 years of follow-up.(15) In the present study, similar data were found. For example, the mean Z score for W/A and H/A, respectively, was −0.27 ± 1.16 and −0.24 ± 1.07.
In the present study, depletion of fat reserve and depletion of muscle mass were found in 10.6% and 16.5% of the patients, respectively. In a study carried out in Florianópolis, Brazil, involving 13 patients between 2 and 20 years of age, 46.1% of the patients presented depletion of muscle mass.(16) The analysis of body composition in CF patients is justified due to the chronic state of catabolic stress or malnutrition related to pulmonary exacerbations which adversely affect energy balance and protein metabolism, principally affecting the body protein reserve.
Good adherence to the nutritional treatment in this sample can also be observed in the energy intake. The mean intake of the 82 evaluated patients was 124.56% of the RDA, and the mean distribution of the nutrients was as follows: 14.6% proteins, 29.9% lipids and 55.2% carbohydrates. One study involving adolescents revealed that boys and girls with normal growth consumed a mean of 110% of the RDA.(17)
Another study demonstrated that the nutrient intake in patients with CF was similar to those recommended for the age and gender.(18) One group of authors found a mean energy intake of 117% of the RDA and percentage of nutrients of 35.7% lipids, 47.9% carbohydrates and 15.2% protein; only 40% of the patients reached the recommended 120% of the RDA.(19) The percentages of macronutrients found in the present study are within the standard range recommended for the population in general, indicating a balance in the distribution of the diet. However, the contribution of foods rich in fat could be slightly higher at the expense of carbohydrates.
Being cross-sectional, the present study only suggests associations. It is impossible to establish a cause-and-effect relationship between food intake and the impairment of the nutritional status.
In the crude analysis, caloric intake was associated only with the nutritional indicator Z score for H/A. The height deficit for the age can represent the presence of intrauterine malnutrition or the chronic presence of malnutrition.(20) There is evidence that, in CF patients, the decrease in height is more important to determining survival than is the decrease in weight for height.(6,21) The effect of low height on survival can be influenced by the lack of growth in a period of life during which there is normally significant growth and pulmonary development.(6)
In the multivariate analysis, adjusted for MRSA bacterial colonization, age, number of hospitalizations and VEF1, a low level of maternal education presented a borderline association with height deficit. In a study of the relationship between maternal caregiving capacity and malnutrition, it was observed that a low level of maternal education doubled the risk for malnutrition.(22) One group of authors observed that less privileged CF patients presented greater impairment of pulmonary function and nutritional status, as well as a 3.65 times higher risk of death.(23) Another recent study demonstrated that patients of higher socioeconomic status presented a 40% lower risk of death when compared with patients of lower socioeconomic status.(24)
However, in the multivariate model, caloric intake was a predictive factor of height in CF patients: when adjusted for age, MRSA bacterial colonization, VEF1, number of hospitalizations and maternal level of education, greater caloric intake was associated with a lower risk of malnutrition due to height deficit. One study demonstrated a significant increase in the Z score for H/A after a nutritional intervention, through which CF patients reached a caloric intake of 132% of the RDA.(25)
The importance of nutritional status in CF patients is well-known and has been widely documented. The present study shows evidence of the association between caloric intake and nutritional status, indicating that dietary profile is a predictor of height in these patients.
AcknowledgmentsWe wish to thank the patients who agreed to participate in the study. We are also grateful for the support provided by the Pulmonology Clinic, Pediatric Pulmonology Department and Nutrition and Dietary Department of the HCPA.
References 1. Dodge JA, Turck D. Cystic fibrosis: nutritional consequences and management. Best Pract Res Clin Gastroenterol. 2006;20(3):531-46.
2. Koletzko S, Reinhardt D. Nutritional challenges of infants with cystic fibrosis. Early Hum Dev. 2001;65 Suppl:S53-61.
3. National Research Council (U.S.). Subcommittee on the Tenth Edition of the RDAs.; National Institutes of Health (U.S.); National Research Council (U.S.). Committee on Dietary Allowances. Recommended dietary allowances. Washington: National Academy Press; 1989.
4. Cystic Fibrosis Foundation. Patient Registry 2004 - Annual date report. Bethesda: Cystic Fibrosis Foundation; 2005.
5. Registro Latino-Americano de Fibrosis Quística (REGLAFQ). Informe del cuarto año. Buenos Aires; 1993. p.21.
6. Beker LT, Russek-Cohen E, Fink RJ. Stature as a prognostic factor in cystic fibrosis survival. J Am Diet Assoc. 2001;101(4):438-42.
7. Zapletal A, Motoyama EK, Gibson LE, Bouhuys A. Pulmonary mechanics in asthma and cystic fibrosis. Pediatrics. 1971;48(1):64-72.
8. Moulin CC, Tiskievicz F, Zelmanovitz T, de Oliveira J, Azevedo MJ, Gross JL. Use of weighed diet records in the evaluation of diets with different protein contents in patients with type 2 diabetes. Am J Clin Nutr. 1998;67(5):853-7.
9. Anção MS, Cuppari L, Tudisco LS, Draibe AS, Sigulem D. Sistema de apoio à decisão em nutrição-versão 2.5. São Paulo: Centro de Informática em Saúde da Universidade Federal de São Paulo/Escola Paulista de Medicina; 1993.
10. Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34(11):2540-5.
11. Sinaasappel M, Stern M, Littlewood J, Wolfe S, Steinkamp G, Heijerman HG, et al. Nutrition in patients with cystic fibrosis: a European Consensus. J Cyst Fibros. 2002;1(2):51-75.
12. Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35(3):246-59.
13. WHO Expert Committee on Physical Status. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. Geneva: The Organization; 1995. WHO Technical Report Series No.: 854.
14. Collins CE, MacDonald-Wicks L, Rowe S, O'Loughlin EV, Henry RL. Normal growth in cystic fibrosis associated with a specialised centre. Arch Dis Child. 1999;81(3):241-6.
15. Morison S, Dodge JA, Cole TJ, Lewis PA, Coles EC, Geddes D, et al. Height and weight in cystic fibrosis: a cross sectional study. UK Cystic Fibrosis Survey Management Committee. Arch Dis Child. 1997;77(6):497-500.
16. Fiates GM, Barbosa E, Auler F, Feiten SF, Miranda F. Estado nutricional e ingestão alimentar de pessoas com fibrose cística. Rev Nutri. 2001;14(2):95-101.
17. Bentur L, Kalnins D, Levison H, Corey M, Durie PR. Dietary intakes of young children with cystic fibrosis: is there a difference? J Pediatr Gastroenterol Nutr. 1996;22(3):254-8.
18. Powers SW, Patton SR, Byars KC, Mitchell MJ, Jelalian E, Mulvihill MM, et al. Caloric intake and eating behavior in infants and toddlers with cystic fibrosis. Pediatrics. 2002;109(5):E75-5.
19. White H, Morton AM, Peckham DG, Conway SP. Dietary intakes in adult patients with cystic fibrosis--do they achieve guidelines? J Cyst Fibros. 2004;3(1):1-7.
20. Vitolo MR. Avaliação nutricional da criança. In: Vitolo MR, editor. Nutrição, da gestação à adolescência. Rio de Janeiro: Reichmann & Affonso; 2003. p. 99.
21. Hayllar KM, Williams SG, Wise AE, Pouria S, Lombard M, Hodson ME, et al. A prognostic model for the prediction of survival in cystic fibrosis. Thorax. 1997;52(4):313-7.
22. Carvalhaes MA, Benício MH. Mother's ability of childcare and children malnutrition [Article in Portuguese]. Rev Saude Publica. 2002;36(2):188-97.
23. Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331-7.
24. O'Connor GT, Quinton HB, Kahn R, Robichaud P, Maddock J, Lever T, et al. Case-mix adjustment for evaluation of mortality in cystic fibrosis. Pediatr Pulmonol. 2002;33(2):99-105.
25. Gaspar MC, Chiba SM, Gomes CE, Juliano Y, Novo NF, Ancona-Lopez F. Resultado de intervenção nutricional em crianças e adolescentes com fibrose cística. J Pediatr (Rio J). 2002;78(2):161-70.
* Study carried out in the Porto Alegre Hospital de Clínicas, Universidade Federal do Rio Grande do Sul - UFRGS, Rio Grande do Sul Federal University - School of Medicine, Porto Alegre, Brazil.
Correspondence to: Míriam Isabel Souza dos Santos Simon. Av. Ramiro Barcelos, 2350, CEP 90035-903, Porto Alegre, RS, Brasil.
Tel 55 51 2101-8410. E-mail: misantos@hcpa.ufrgs.br
Financial support: This study received financial support from the Fundo de Incentivo à Pesquisa (FIPE, Research Incentive Fund) and from the Grupo de Pós-Graduação e Pesquisa (GPPG, Postgraduate and Research Group) of the Porto Alegre Hospital de Clínicas.
Submitted: 3 September 2008. Accepted, after review: 1 June 2009.
About the authorsMíriam Isabel Souza dos Santos Simon
Head of the Nutrition and Dietary Department. Porto Alegre Hospital de Clínicas, Universidade Federal do Rio Grande do Sul - UFRGS, Rio Grande do Sul Federal University - School of Medicine, Porto Alegre, Brazil.
Michele Drehmer
Doctoral Student in Epidemiology. Universidade Federal do Rio Grande do Sul - UFRGS, Rio Grande do Sul Federal University - Porto Alegre, Brazil.
Sérgio Saldanha Menna-Barreto
Full Professor. Department of Internal Medicine, Universidade Federal do Rio Grande do Sul - UFRGS, Rio Grande do Sul Federal University - School of Medicine, Porto Alegre, Brazil.





