A 68-year-old Brazilian woman was admitted to our ICU with sudden and intense shortness of breath and acute chest pain. She had a history of COPD and was under treatment with home oxygen therapy and bronchodilators. She had presented severe bronchospasm and required large doses of inhaled 2 adrenergic agonist at home. On physical examination, blood pressure was 142 × 54 mmHg and HR was 132 bpm. She presented with diffuse wheezing, bibasilar rales, and decreased peripheral perfusion.
The electrocardiogram revealed sinus tachycardia with decreased septal QRS vector, intraventricular conduction disturbance, diffuse T-wave inversions, and corrected QT (QTc) of 543 ms (Figure 1). The troponin T peak and creatine kinase-MB peak were 2.5 ng/mL (normal value < 0.26 ng/mL) and 17.6 ng/mL (normal value < 3.6 ng/mL), respectively. The patient was treated as having high-risk acute coronary syndrome with acetylsalicylic acid, nitroglycerin, and intravenous heparin. Transthoracic echocardiogram with limited echocardiographic window showed hypokinesia of the mid-apical segments of the anterior, lateral, septal, and inferior walls (Figures 2a and 2b); ejection fraction of 25%; and diastolic dysfunction with impaired relaxation. The left ventricular (LV) configuration resembled the shape of a Japanese fishing pot with a rounded bottom and a narrow neck, used for trapping octopuses, known as a takotsubo, from which the term "takotsubo cardiomyopathy" is derived.
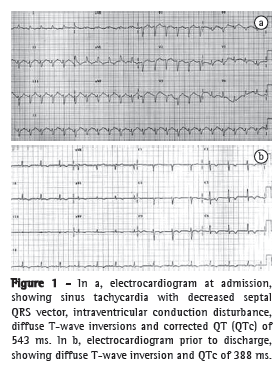

Six hours after admission, the patient developed cardiogenic shock. She was given large doses of catecholamines (dobutamine and noradrenalin), was submitted to endotracheal intubation, and required mechanical ventilation. She underwent emergency cardiac catheterization, which showed that her coronary arteries were normal. Cardiogenic shock reverted within 48 h, with an LV ejection fraction of 60% (Figures 2c and 2d). After a follow-up period of 2 years, she had normal LV function and QTc of 388 ms (Figure 1), without major adverse cardiac events.
Takotsubo cardiomyopathy is characterized by transient LV systolic dysfunction in one or more segments. It is more prevalent in women (6-9:1), individuals over 60 years of age, and Whites (when compared with Asians).(1) The clinical presentation can be chest pain, dyspnea, syncope, tachyarrhythmia/bradyarrhythmia, and heart failure. Precipitating events are usually intense physical or mental stress or an acute illness.
One study demonstrated a favorable prognosis regarding the improvement of the LV function, with an in-hospital mortality rate of 1%.(2) The reported rate of complications is 18.9%, such complications including cardiogenic shock, in 6.5%; thrombus, in 3.8%; cardiac failure, in 3.8%; and death, in 3.2%.(1)
The pathophysiology is unknown, and it has been related to catecholamine-induced myocardial dysfunction, neurally mediated myocardial stunning, abnormalities in coronary endothelial function, multiple vasospasms of the epicardial coronary arteries, or microvascular dysfunction.(2,3)
Transient cardiomyopathy resulting from the administration of exogenous catecholamines (beta agonists and methylxanthines) has been reported in patients presenting with exacerbations of underlying asthma. The use of high doses of inhaled beta agonist bronchodilators in those with acute pulmonary disease might lead to adrenergic overstimulation of the heart with the potential for catecholamine cardiotoxicity. Exaggerated sympathetic stimulation is probably related to the pathophysiology of takotsubo cardiomyopathy and could lead to transient microvascular dysfunction with LV wall motion abnormalities in multiple segments.(4,5) Excessive systemic release of catecholamines probably exerts a paradoxical negative inotropic effect on myocytes via 2 adrenergic receptors in the apical region, where the density of such receptors is the greatest.(6) There is also a direct effect of high catecholamine concentrations via a cAMP-mediated calcium overload, leading to contraction band necrosis, which reduces myocyte contractility. In addition, the striking preponderance of postmenopausal women presenting takotsubo cardiomyopathy indicates that estrogen deficiency plays a role in its pathogenesis.
This case was unusual in various aspects. Initially, the triggering factors were exacerbation of bronchial asthma and use of large doses of beta agonists. She then developed cardiogenic shock, becoming dependent on inotropes. The patient met all of the Mayo Clinic diagnostic criteria for takotsubo cardiomyopathy, which include transient LV contractile dysfunction, extending beyond the territory of a single epicardial coronary artery; absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; the development of abnormalities on electrocardiogram or elevated cardiac troponin; and absence of pheochromocytoma or myocarditis.(7) Due to massive catecholamine release, it is recommended that neither large doses of beta mimetics (for treating severe asthma exacerbations) nor sympathomimetics be used. Prolonged use of adrenaline-like substances with inotropic effects can increase the already high circulating levels and augment oxygen consumption, worsening myocardial stunning. This is also associated with the overproduction of oxygen-derived free radicals in these patients.
Sympathomimetics can also lead to further hemodynamic deterioration, in particular when the patient presents an intraventricular pressure gradient (IVPG), which increases mid-ventricular obstruction. The use of beta-blockers with careful hemodynamic monitoring might be indicated in patients with both takotsubo cardiomyopathy and IVPG. In cases of LV thrombus, atrial fibrillation, or systemic embolism, the use of anticoagulation is also indicated.
Vera Maria Cury Salemi
Cardiologist, Hospital Sírio Libanês Cardiology Center, São Paulo, Brazil
Edmar Atik
Cardiologist, Hospital Sírio Libanês Cardiology Center, São Paulo, Brazil
Ronaldo Adib Kairalla
Pulmonologist, Hospital Sírio Libanês Cardiology Center, São Paulo, Brazil
Eduardo Lira Queiroz
Pulmonologist, Hospital Sírio Libanês Cardiology Center, São Paulo, Brazil
Leonardo Vieira da Rosa
Cardiologist, Hospital Sírio Libanês Cardiology Center, São Paulo, Brazil
Roberto Kalil Filho
Director, Hospital Sírio Libanês Cardiology Center, São Paulo, BrazilReferences1. Donohue D, Movahed MR. Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev. 2005;10(4):311-6.
2. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858-65.
3. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523-9.
4. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-48.
5. Kume T, Kawamoto T, Okura H, Toyota E, Neishi Y, Watanabe N, et al. Local release of catecholamines from the hearts of patients with tako-tsubo-like left ventricular dysfunction. Circ J. 2008;72(1):106-8.
6. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5(1):22-9.
7. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408-17.



