ABSTRACT
Objective: To determine the prevalence of pulmonary hypertension (PH) in patients with cystic fibrosis (CF), to compare clinical
characteristics, radiographic scores, pulmonary function, and echocardiographic parameters in patients with and without PH, and to correlate
echocardiographic findings with clinical characteristics, radiographic scores, and pulmonary function. Methods: This was a prospective,
cross-sectional study involving clinically stable patients (aged 16 or older) enrolled in an adult CF program. The patients were submitted to
clinical evaluation, Doppler echocardiography, pulmonary function tests, and chest X-rays. Results: Tricuspid regurgitant jet velocity (TRV)
was obtained in 37 of the 40 patients studied. The prevalence of PH was 49% with a TRV cut-off of 2.5 m/s (18 patients) and 30% with
a TRV cut-off of 2.8 m/s (11 patients). Peripheral oxygen saturation (SpO2) at rest, clinical score, forced expiratory volume in one second
(FEV1), and forced vital capacity (FVC) were significantly lower in the group with PH. The TRV was found to correlate significantly with SpO2
at rest (p < 0.001), clinical score (p < 0.001), radiographic score (p = 0.030), FEV1 in liters (p < 0.001) and in % of predicted (p < 0.001), and
FCV in liters (p = 0.008) and in % of predicted (p = 0.001). The single best predictor of TRV was SpO2 at rest (p < 0.001). Conclusion: The
high prevalence of PH in the CF patients studied suggests that PH should be considered in the evaluation and follow-up treatment of such
patients. The best predictor of PH was SpO2 at rest.
Keywords:
Cystic fibrosis; Tricuspid valve/physiopathology; Hypertension, pulmonary; Echocardiography, Doppler.
RESUMO
Objetivo: Determinar a prevalência de hipertensão pulmonar (HP) em pacientes com fibrose cística (FC), comparar características clínicas,
escore radiológico, função pulmonar e parâmetros ecocardiográficos nos grupos com e sem HP e correlacionar achados ecocardiográficos
com características clínicas, escore radiológico e função pulmonar. Métodos: Estudo transversal prospectivo em pacientes clinicamente estáveis
(idade ≥ 16 anos) atendidos por um programa de adultos para FC. Os pacientes foram submetidos a avaliação clínica, ecocardiografia
Doppler, testes de função pulmonar e exame radiológico do tórax. Resultados: Obteve-se a velocidade de regurgitação tricúspide (VRT) em
37 dos 40 pacientes estudados. A prevalência de HP foi de 49% com um ponte de corte da VRT de 2,5 m/s (18 pacientes) e de 30% com um
ponte de corte da VRT de 2,8 m/s (11 pacientes). Os valores de saturação periférica de oxigênio (SpO2) em repouso, escore clínico, volume
expiratório forçado no primeiro segundo (VEF1) e capacidade vital forçada (CVF) foram significativamente menores no grupo com HP. A VRT
correlacionou-se significativamente com SpO2 em repouso (p < 0,001), escore clínico (p < 0,001), escore radiológico (p = 0,030), VEF1 em
litros (p < 0,001) e em % do previsto (p < 0,001) e CVF em litros (p = 0,008) e em % do previsto (p = 0,001). A SpO2 em repouso foi o melhor
preditor independente da VRT (p < 0,001). Conclusão: A alta prevalência de HP nos pacientes com FC estudados sugere que a presença de
HP seja considerada na avaliação e acompanhamento desses pacientes. O melhor preditor de HP foi a SpO2 em repouso.
Palavras-chave:
Fibrose cística; Valva Tricúspide/fisiopatologia; Hipertensão pulmonar; Ecocardiografia Doppler.
Introduction Cystic fibrosis (CF) is an autosomal recessive genetic disease of chronic, progressive, and irreversible evolution. It is clinically identified by the triad consisting of chronic suppurative obstructive pulmonary disease, pancreatic insufficiency, and high levels of sweat electrolytes.(1,2)
Although CF is multisystemic in nature, pulmonary involvement is the main determinant of morbidity and mortality. As the lung disease progresses, a large percentage of patients with CF develop pulmonary hypertension (PH). In such patients, the development of alveolar hypoxia (in hypoventilated areas) leads to hypoxic pulmonary vasoconstriction. When the hypoxic state is prolonged, the pulmonary circulation suffers structural alterations collectively known as remodeling, characterized by hypertrophy and hyperplasia of the arterial media, as well as by muscle fibers in the peripheral vessels.(3-5)
The diagnostic procedure that is the most accurate in identifying the onset of PH is heart catheterization, and the most sensitive noninvasive technique for the early detection and monitoring of PH is Doppler echocardiography.(4,5)
Echocardiography allows us to identify and quantify PH, as well as to determine its variability and the repercussions for the right heart chambers. In addition, echocardiography allows serial evaluations after therapeutic interventions. Common findings in the evaluation of patients with PH include dilatation of the right heart chambers, right ventricular hypertrophy, paradoxical septal movement, and tricuspid insufficiency.(4-7)
According to some studies,(8,9) a large percentage of patients with CF present subclinical PH. The great phenotypic variability of the disease(10) justifies a systematic diagnostic approach to evaluate PH in a population of adolescent and adult patients in Brazil. In addition, quantitative parameters of routine clinical practice, such as clinical score, radiographic score, peripheral oxygen saturation (SpO2), and spirometric variables, might indicate the appropriate time point at which to test for PH in such patients. This possibility motivates further study of the issue.
Therefore, the objective of the present study was to determine, using Doppler echocardiography, the prevalence of PH in patients enrolled in an adult CF program, as well as to compare clinical characteristics, radiographic scores, pulmonary function, and echocardiographic parameters in patients with and without PH. In addition, we attempted to determine whether indicators of right ventricular function correlate with clinical characteristics, radiographic score, and pulmonary function.
Methods This was a prospective, cross-sectional study. The study population comprised patients diagnosed with CF, in accordance with the consensus criteria,(11) enrolled in the adult program of the Hospital de Clínicas de Porto Alegre (HCPA, Porto Alegre Hospital de Clínicas). The inclusion criteria were being at least 16 years of age and being clinically stable. The exclusion criterion was presenting primary heart disease. Initially, each patient underwent a clinical evaluation in order to determine clinical stability and clinical CF score. Subsequently (within a period of one week), the patients underwent Doppler echocardiographic studies, pulmonary function tests, and conventional chest X-rays.
All patients underwent transthoracic two-dimensional M-mode Doppler echocardiography (ATL-HDI 5000; Philips, Bothell, WA, USA). The echocardiographic studies were performed, through the standardization of the parasternal, apical, and subcostal windows with the patient at rest, by a single observer who was blinded as to patient clinical status. The echocardiographic measurements were performed in accordance with the American Society of Cardiology guidelines.(12) Tricuspid regurgitation was determined by the backflow of blood across the tricuspid valve at each contraction of the right ventricle, as seen on the apical parasternal view of the four chambers. The Bernoulli equation was used to estimate the systolic pressure gradient based on the peak tricuspid regurgitant jet velocity (TRV):
Δp = 4v 2
where Δp corresponds to delta pressure, and v corresponds to velocity. For the purpose of analysis, PH was defined as a TRV > 2.5 m/s. A more conservative TRV cut-off point (2.8 m/s) was also used to determine the prevalence of PH. By tracing the anterograde pulmonary flow, we calculated the right ventricular/pulmonary artery systolic acceleration time (SAT), which represents the interval between the onset of the flow in the pulmonary artery and the peak flow velocity. The right ventricular diameter (RVD) was measured at the end of the diastole.
The dimensions of the left chambers were obtained, through the parasternal window, using the M-mode. The ejection fraction was evaluated using the Teichholz method. The left ventricular mass was calculated using the modified Devereux formula, corrected for body surface area and expressed as a mass index. The left atrial diameter was determined, at the end of the left ventricular ejection, by measuring the vertical distance between the inner surface of the posterior wall of the aorta and the posterior wall of the atrium.
The Shwachman-Kulczycki clinical evaluation score was used.(13) This clinical evaluation system considers four different characteristics (general activity, physical examination, nutrition, and chest X-ray findings), each of which is scored on a scale ranging from 5 to 25 points.
A conventional chest X-ray was performed in all of the individuals evaluated. A single pulmonologist, who was blinded as to patient clinical status and patient identity, determined the radiographic scores using the radiographic scoring system developed by Brasfield et al.(14) The following characteristics were scored, listed here in ascending order by degree of severity: air trapping (0 to 4); linear markings (0 to 4); nodulocystic lesions (0 to 4); extensive lesions in the air space (0, 3, or 5); and overall severity (0 to 5). The total score was 25 minus the total score obtained in the five characteristics examined.
The pulmonary function tests were performed using a computerized spirometer (Jaeger-v4.31; Jaeger, Würzburg, Germany). Three maneuvers were performed, and the value for the best of the three was registered. The parameters studied were forced vital capacity (FVC) in liters and in percentage of predicted, and forced expiratory volume in one second (FEV1) in liters and in percentage of predicted for age, height, and gender.(15) Airflows were analyzed in accordance with the Pulmonary Function Test Guidelines established by the Brazilian Thoracic Society.
Evaluation of peripheral oxygen saturation (SpO2) was performed with the patient at rest and using a model NPB-40 pulse oximeter (Nellcor Puritan Bennett, Pleasanton, CA, USA).
Data were entered into a Microsoft Excel 2000 database, after which they were analyzed using the Statistical Package for the Social Sciences program, version 13.0. Quantitative data are expressed as mean ±standard deviation or as median and interquartile range. Qualitative data are expressed as number and percentage of all cases.
Quantitative data with normal distribution were analyzed using the t-test for independent samples. Continuous data with non-normal distribution were analyzed using Mann-Whitney U test. Qualitative data were analyzed using the chi-square test. Pearson's linear correlation test was used for variables with normal distribution, and Spearman's correlation coefficient was used for variables with non-normal distribution. Multiple linear regression analysis was performed for the independent variables that were associated with TRV. The level of statistical significance was set at p < 0.05.
Results In the period from September of 2004 to May of 2006, 40 of the 41 patients enrolled in the adult CF program at the HCPA were included in the study. One patient refused to participate in the study. A total of 22 females and 18 males were studied. The mean age was 23.7 ±6.3 years, and the mean TRV was 2.5 ± 0.3 m/s.
Table 1 summarizes the clinical, pulmonary function, radiographic, and echocardiographic characteristics of the patients by TRV value. Nineteen patients presented TRV values ≤2.5 m/s and were classified as not having PH, whereas 18 patients presented TRV values > 2.5 m/s and were classified as having PH. The values of FEV1 and FVC in % of predicted were significantly lower in the group with PH than in the group without PH (p < 0.001 and p = 0.001, respectively). The values of SpO2 at rest and at the end of the six-minute walk test (6MWT) were significantly lower in the group with PH than in the group without PH (p < 0.001 and p = 0.007, respectively). The RVD values differed significantly between the two groups (p = 0.023). The left ventricular ejection fraction ranged from 54 to 76% in the sample as a whole, and there was no significant difference between the two groups. In the analysis using the more conservative TRV cut-off point, 11 of the 37 patients presented TRV values ≥ 2.8 m/s (above the cut-off).

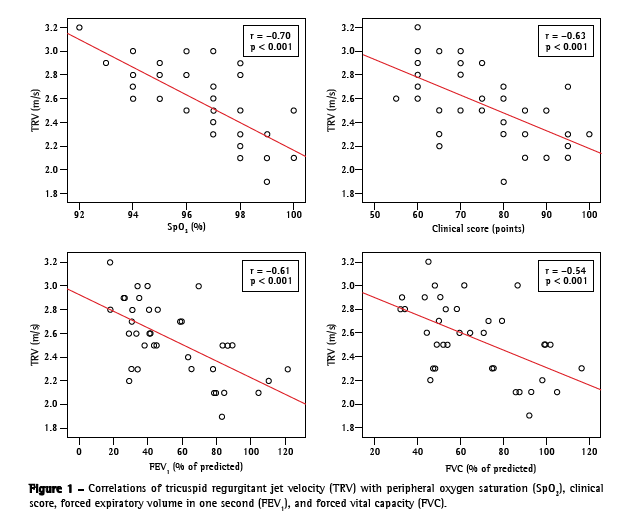
Figure 1 shows the graphic representation of how TRV correlated with SpO2, clinical score, FEV1 in % of predicted, and FVC in % of predicted. A significant correlation was found between TRV and SpO2 at rest [r = −0.70 (−0.80; −0.50); p < 0.001]. In addition, TRV was found to correlate significant with the following variables: post-6MWT SpO2 [r = −0.47 (−0.68; −0.19); p = 0.003]; clinical score [r = −0.63 (−0.79; −0.40); p < 0.001]; radiographic score [r = −0.36 (−0.60; −0.06); p = 0.030]; FEV1 in liters [r = −0.55 (−0.74; −0.29); p < 0.001]; FEV1 in % of predicted [r = −0.61 (−0.77; −0.37); p < 0.001]; FVC in liters [r = −0.43 (−0.65; −0.14); p = 0.008]; and FVC in % of predicted [r = −0.54 (−0.73; −0.28); p = 0.001]. The SAT was found to correlate with clinical score [r = 0.37 (0.07; 0.61); p = 0.020], as well as with FEV1 in % of predicted [r = 0.35 (0.04; 0.60); p = 0.028] and FVC [r = 0.34 (0.03; 0.59); p = 0.037] in % of predicted. The RVD was found to correlate with SpO2 [r = −0.44 (−0.66; −0.15); p = 0.004], clinical score [r = −0.38 (−0.62; −0.08); p = 0.015], and FEV1 in % of predicted [r =−0.33 (−0.58; −0.02); p = 0.037].
In the multiple linear regression analysis (stepwise method), clinical score, FEV1, radiographic score, and SpO2 at rest, all adjusted for gender and age, were included as independent variables. It was found that SpO2 at rest was the best predictor of TRV, and that its effect was independent of the associations among other independent variables (p < 0.001).
Discussion In the present study, by evaluating patients enrolled in an adult CF program (40 of the 41 patients enrolled in the program), regardless of the severity of the pulmonary disease, and using Doppler echocardiography as a diagnostic tool, we showed that the prevalence of PH in this population was 49% when a TRV cut-off point of 2.5 m/s was used and 30% when a TRV cut-off point of 2.8 m/s was used.
In our study, TRV was used as a PH-defining variable, in contrast with previous studies.(8,9) The patients classified as having PH presented significantly lower clinical scores, as well as lower values for FEV1, FVC, and SpO2 (at rest and at the end of the 6MWT), than did the patients classified as not having PH. The TRV correlated inversely with
Prevalence of pulmonary hypertension evaluated by Doppler echocardiography in a population of adolescent and adult patients with cystic fibrosis the markers of pulmonary disease severity (SpO2 at rest, clinical score, radiographic score, FEV1, and FVC), whereas the SAT and the RVD correlated more weakly with these variables.
The noninvasive method of echocardiographic assessment of pulmonary artery pressures is based on the TRV.(16) The TRV reflects the difference between right ventricular and right atrial pressure and can be calculated using the Bernoulli equation.(12) When the estimated right atrial pressure is added to that gradient,systolicrightventricularpressureisobtained. The results obtained using this method, which is simple and easily applied, have correlated well with those from invasive pulmonary artery pressure measurements in hemodynamics laboratory tests.(17-19)
There have been few studies evaluating PH in patients with CF. The principal ones are mentioned below.
One group of authors(8) studied 18 adult CF patients with severe pulmonary disease in order to achieve the following objectives: to determine the prevalence of PH and cardiac dysfunction; to study the relationship between cardiovascular abnormalities and hypoxemia; and to evaluate the impact of subclinical PH on survival. Of the 18 patients evaluated, 7 (39%) were found to have PH. Pulmonary artery systolic pressure (PASP) was found to correlate significantly with FEV1, waking SpO2, SpO2 during sleep, and SpO2 after exercise. In the multivariate analysis, waking SpO2 was the best predictor of PASP. The five-year clinical follow-up evaluation of that cohort showed that mortality was significantly higher in the patients with PH. As previously mentioned, in our study, all patients were evaluated, regardless of the severity of the pulmonary disease. The 49% prevalence of PH identified in our study reveals that even patients with less severe pulmonary disease can present PH. Similar to what was found in the study mentioned above, linear regression showed that SpO2 at rest was an independent predictor, and the only predictor, of PASP.
In another study,(9) three groups of volunteers were studied: 21 patients with CF and clinically stable disease (Group 1); 5 patients with CF and extremely advanced disease (Group 2); and 23 age- and gender-matched healthy individuals (Group 3). The objective of the study was to evaluate right ventricular function using tissue Doppler echocardiography. In the three groups, the means of the right ventricular (RV) free wall systolic velocity were found to be 8.9 ±1.7 cm/s, 7.7 ±1 cm/s, and 10.8 ±1.9 cm/s, respectively (p < 0.001). The tricuspid annular velocities were significantly lower in the CF patients than in the healthy individuals. The CF patients presented longer isovolumetric relaxation time, indicating RV diastolic dysfunction. The RV wall thickness was greater in the CF patients than in the healthy individuals (p < 0.01). It was concluded that the patients with CF presented subclinical RV dysfunction, which was found to correlate with the severity of the pulmonary disease. In contrast, our study did not use a control group. We also found that the RV wall thickness was significantly greater in the patients who presented a TRV > 2.5 m/s, that is, in the group with PH.
Another group of researchers used echocardiography to evaluate the degree of PH in 45 CF patients (from 3 to 24 years of age).(20) The authors identified increases in the RV end-diastolic dimension (in 67% of the patients), the RV anterior wall thickness (in 71%), and the PASP (in 84%). It was concluded that the right ventricle undergoes significant alterations as the disease progresses, and that echocardiography can be useful in the clinical monitoring of disease severity. The fact that the prevalence of PH in that study was higher that that found in our study might be attributable to differences in sample selection, those authors selecting patients with more severe disease.
In the present study, TRV was found to correlate significantly with FEV1 and FVC (Figure 1). The patients presenting a TRV > 2.5 m/s had significantly lower values of FEV1 and FVC. Other researchers(8) estimated that more than 40% of the adult CF patient population with severe pulmonary disease has PH without clinical signs of cor pulmonale. Those authors found the incidence of PH to be lower among patients with better pulmonary function (FEV1 > 40% of predicted).
The greatest limitation of the present study was the method of evaluating PH. The gold standard method for diagnosing PH is heart catheterization, which, ideally, should have been used. However, it was decided that the measurement would be performed using the noninvasive method since it has been shown to present a satisfactory degree of concordance with the invasive method.(9) Doppler echocardiography is commonly used to estimate PASP and diagnose PH in patients with advanced pulmonary disease. Nevertheless, due to the great variability of this method in estimating PASP in patients with chronic pulmonary disease, some authors have questioned its use for this purpose. (21,22) One group of authors studied a cohort of 374 lung transplant candidates who underwent Doppler echocardiography and right heart catheterization to determine PASP and diagnose PH.(21) The correlation between the PASP estimated by echocardiography and the PASP measured by catheterization was strong (r = 0.69; p < 0.0001). However, 52% of the echocardiographic PASP estimates differed from the PASP measurements performed by catheterization by more than 10 mmHg. Echocardiography also produced false-positive PH results in 48% of the patients. The sensitivity, specificity, positive predictive value, and negative predictive value of the PASP estimate for the diagnosis of PH were, respectively, 85, 55, 52, and 87%. It was concluded that the PASP echocardiographic estimates for the diagnosis of PH are often inaccurate in patients with advanced pulmonary disease and result in the overestimation of this diagnosis. Despite this limitation, Doppler echocardiography, in comparison with right heart catheterization, has the advantage of being a noninvasive procedure and of being easier to use in clinical practice, especially in repeated evaluations. The TRV cut-off point for the definition of PH is not well defined in the literature. Most studies have presented a TRV cut-off point of
2.5 m/s to define PH in patients with diseases such as sickle cell anemia.(17,23-25) The more conservative TRV cut-off point (2.8 m/s) should be employed in cases in which the diagnosis of PH has not been confirmed, since the use of a very low TRV cut-off point could result in the overestimation of the prevalence of PH in certain populations. In addi-
Prevalence of pulmonary hypertension evaluated by Doppler echocardiography in a population of adolescent and adult patients with cystic fibrosis
tion, some studies(8,9,23) have demonstrated that the identification of patients as having PH by Doppler echocardiography is directly associated with poor survival, and this justifies its use at least as a screening method.
Other limitations of the method, as used in our study, were expressly stated. For example, in one case, it was not possible to measure SAT, a fact that was attributed to lung hyperinflation. Furthermore, tricuspid regurgitation was minimal in 3 patients, and it was not possible to measure it using the diagnostic method employed. Those 3 patients were excluded from the study analysis.
Finally, the fact that the study design was cross-sectional prevented us from drawing prognostic conclusions regarding the identification of PH in our patient sample.
The present study, carried out in a referral center for CF, evaluated the prevalence of PH in the patients enrolled in the adult CF program. In contrast to previous studies,(8,9) in which only the most severe patients were selected for inclusion, our study included the vast majority of the patients under follow-up treatment, thus allowing a better estimate of this prevalence. A high prevalence of PH was identified, even in patients in whom SpO2 at rest was still normal. It might therefore be useful to monitor PH in such patients. In order to establish an association between identification of PH in CF and poor prognosis,(8,9) better evaluation and a more intensive follow-up treatment of this group of patients is required.
In conclusion, the present study showed that the prevalence of PH in the patients treated via an adult CF program was 49% when a TRV cut-off point of 2.5 m/s was used and 30% when a more conservative TRV cut-off point was used. This suggests that the PH should evaluated in such patients and should be monitored during their follow-up treatment. The group classified as having PH presented significantly lower clinical scores, as well as significantly lower values of SpO2 at rest, FEV1, and FVC, than did the group classified as not having PH, which highlights the use of these parameters as potential predictors of PH in this population.
Acknowledgments We would like to thank Vânia Naomi Hirakata and Daniela Benzano for the statistical analysis.
We would also like to thank all of the staff of the HCPA Adolescent and Adult CF Program for their collaboration.
References 1. Ratjen F, Döring G. Cystic fibrosis. Lancet. 2003;361(9358):681-9.
2. Robinson P. Cystic fibrosis. Thorax.;56(3):237-41.
3. Camargos PA, Queiroz MV. Pico do fluxo expiratório na avaliação da função pulmonar na fibrose cística. Jornal de Pediatria. 2002;77(1):45-9.
4. Chemla D, Castelain V, Hervé P, Lecarpentier Y, Brimioulle S. Haemodynamic evaluation of pulmonary hypertension. Eur Respir J. 2002;20(5):1314-31.
5. West JB. Doenças ambientais. In: West JB, editor. Fisiopatologia Pulmonar Moderna. 4th ed. São Paulo: Manole; 1996. p. 141-2.
6. Sociedade Brasileira de Pneumologia e Tisiologia. Sociedade Brasileira de Reumatologia. Classificação e avaliação diagnóstica da hipertensão pulmonar. J Bras Pneumol. 2005;31(2):S1-S8.
7. Menna Barreto SS, Gazzana MB. Hipertensão pulmonar: relato de seis casos e atualização do tema. J Pneumol. 2000;26(6):321-36.
8. Fraser KL, Tullis DE, Sasson Z, Hyland RH, Thornley KS, Hanly PJ. Pulmonary hypertension and cardiac function in adult cystic fibrosis: role of hypoxemia. Chest. 1999;115(5):1321-8.
9. Ionescu AA, Ionescu AA, Payne N, Obieta-Fresnedo I, Fraser AG, Shale DJ. Subclinical right ventricular dysfunction in cystic fibrosis. A study using tissue Doppler echocardiography. Am J Respir Crit Care Med. 2001;163(5):1212-8.
10. Gilljam M, Ellis L, Corey M, Zielenski J, Durie P, Tullis DE. Clinical manifestations of cystic fibrosis among patients with diagnosis in adulthood. Chest. 2004;126(4):1215-24.
11. Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125(1 Suppl):1S-39S.
12. Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA; Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167-84.
13. Shwachman H, Kulczycki LL. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J Dis Child. 1958;96(1):6-15.
14. Brasfield D, Hicks G, Soong S, Tiller RE. The chest roentgenogram in cystic fibrosis: a new scoring system. Pediatrics. 1979;63(1):24-9.
15. Pereira CA, Barreto SP, Simões JG, Pereira FW, Gerstler JG, Nakatani J. Valores de referência para espirometria em uma amostra da população brasileira adulta. J Pneumol. 1992;18(1):10-22.
16. Sbano JC, Tsutsui JM, Terra-Filho M, Mathias Junior W. Papel da ecodopplercardiografia na avaliação da hipertensão pulmonar. J Bras Pneumol. 2004;30(1):78-86.
17. Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6(2):359-65.
18. Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6(4):750-6.
19. Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70(4):657-62.
20. Podolska E, Pogorzelski A, WośH. [Echocardiographic assessment of cor pulmonale in patients with cystic fibrosis] [Article in Polish]. Wiad Lek. 2006;59(3-4):208-13.
21. Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167(5):735-40.
22. Laaban JP, Diebold B, Zelinski R, Lafay M, Raffoul H, Rochemaure J. Noninvasive estimation of systolic pulmonary artery pressure using Doppler echocardiography in patients with chronic obstructive pulmonary disease. Chest. 1989;96(6):1258-62.
23. Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886-95.
24. Machado RF, Martyr S, Kato GJ, Barst RJ, Anthi A, Robinson MR, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130(3):445-53.
25. Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, et al. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296(3):310-8.
___________________________________________________________________________________________________________________
Study carried out in the Department of Pulmonology of the Porto Alegre Hospital de Clínicas, Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - Porto Alegre, Brazil.
1. Assistant Professor at the School of Physical Therapy. Methodist University Center Instituto Porto Alegre - IPA, Porto Alegre Institute - Porto Alegre, Brazil.
2. Physical Therapist in the Department of Pulmonology. Porto Alegre Hospital de Clínicas, Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - Porto Alegre, Brazil.
3. Cardiologist/Echocardiographer in the Department of Cardiology. Porto Alegre Hospital de Clínicas. Porto Alegre, Brazil.
4. Full Professor in the Department of Internal Medicine. Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - School of Medicine, Porto Alegre, Brazil.
5. Adjunct Professor in the Department of Internal Medicine. Universidade Federal do Rio Grande do Sul - UFRGS, Federal University of Rio Grande do Sul - School of Medicine, Porto Alegre, Brazil.
Correspondence to: Paula Maria Eidt Rovedder. Rua Domingos Crescêncio, 185/502, Bairro Santana, CEP 90650-090, Porto Alegre, RS, Brasil.
Tel 55 51 3210-8241. E-mail: larove_@hotmail.com
Submitted: 21 January 2007. Accepted, after review: 13 June 2007.



