BACKGROUND Lung transplantation significantly affects various domains of respiratory physiology. Some changes result from the procedure itself and any direct lung injury related to it. Post-transplantation chronic lung allograft dysfunction (CLAD) remains a major cause of morbidity and mortality.(1) Therefore, lung transplant recipients should undergo regular pulmonary function tests (PFTs) as part of ongoing monitoring.(1,2)
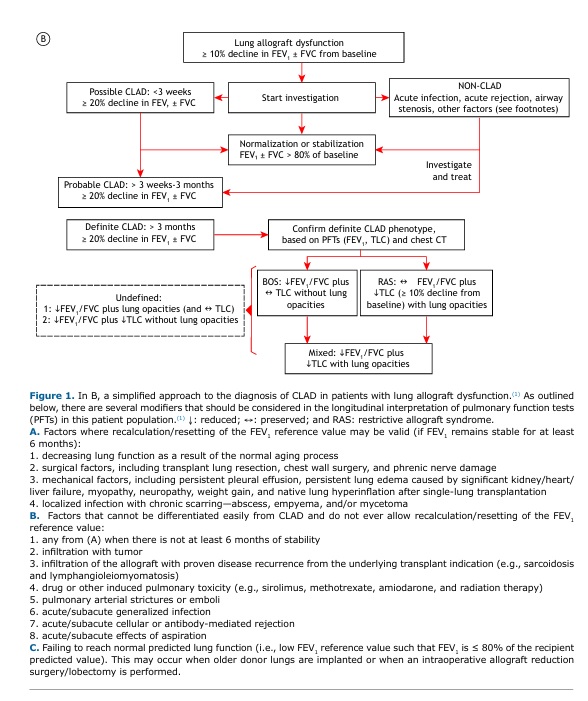
OVERVIEW A 60-year-old woman underwent right lung transplantation because of severe emphysema. Despite mild acute lung rejection in the first post-transplant year, she remained largely asymptomatic with relatively preserved lung function over several years. However, she reported progressive exertional dyspnea after a severe lower respiratory tract infection six years later. Despite treatment optimization, there had been a persistent (> 3 months) decline in FEV1 (≥ 20%) relative to baseline (Figure 1A). The presence of obstruction (low FEV1/FVC) without restriction (preserved TLC) or new opacities on chest CT suggested the obstructive phenotype of CLAD (Figure 1B).(1)
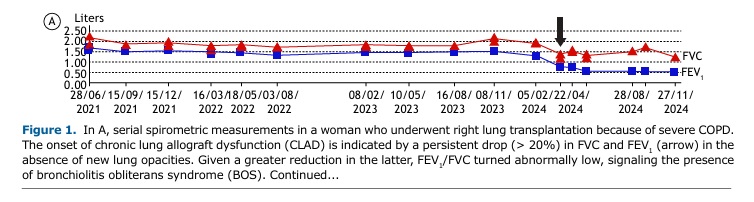
Changes in PFTs after lung transplantation are influenced by the underlying lung disease of the recipient and whether the transplant is single or bilateral.(3,4) Clinical interpretation of PFTs in recipients of single transplants is more complex because changes may reflect the progression of the underlying disease in the native lung. Most centers recommend (at least) spirometry once a month for the first post-transplant year and every 3-4 months subsequently. FVC and FEV1 usually improve over the first three months following surgery, and there is a slight further improvement up to 24 months after bilateral transplantation.(3,4) The average of two maximal post-transplant FEV1 values obtained at least three weeks apart should be recorded as a baseline for monitoring allograft function.(1) Supranormal FEV1/FVC might be seen, secondary to a restrictive thoracic cage due to the operative procedure and/or transplantation of large lungs, causing a mismatch between higher airflow capacity and thoracic cage volume. A persistent (> 2 days) decline of 10% in spirometric values has been reported to indicate either rejection or infection.(5)
CLΑD is an umbrella term describing a significant decline in lung function after lung transplantation in the absence of other identifiable causes. The most common manifestation of CLAD is bronchiolitis obliterans syndrome. However, up to 30% of patients with CLAD develop a restrictive phenotype. A diagnostic workup is provided in Figure 1B. More sensitive metrics of smaller airway dysfunction (such as low mid-expiratory flows and impulse oscillometry measurements) and/or air trapping (high functional residual capacity and RV) are not widely considered given the great variability and the lack of data from large studies examining this issue. However, persistent changes in these parameters and those reflecting impaired gas transfer (hemoglobin-corrected DLCO and carbon monoxide transfer coefficient) might be relevant in individual subjects.
CLINICAL MESSAGE PFTs are critical for monitoring allograft (dys)function; for early detection of rejection and infection; and for monitoring response to treatment. Careful clinical and imaging correlation is paramount. Close attention should be given to factors that can negatively impact lung function, such as weight gain, aging, comorbidities, and concurrent local or systemic pathological processes (Figure 1, footnotes).
AUTHOR CONTRIBUTIONS All authors contributed equally to this manuscript.
CONFLICTS OF INTEREST None declared.
REFERENCES 1. Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019;38(5):493-503. https://doi.org/10.1016/j.healun.2019.03.009
2. Sheshadri A, Sacks NC, Healey BE, Raza S, Boerner G, Huang HJ. Lung Function Monitoring After Lung Transplantation and Allogeneic Hematopoietic Stem Cell Transplantation. Clin Ther. 2022;44(5):755-765.e6. https://doi.org/10.1016/j.clinthera.2022.03.011
3. Belloli EA, Wang X, Murray S, Forrester G, Weyhing A, Lin J, et al. Longitudinal Forced Vital Capacity Monitoring as a Prognostic Adjunct after Lung Transplantation. Am J Respir Crit Care Med. 2015;192(2):209-18. https://doi.org/10.1164/rccm.201501-0174OC
4. Pochettino A, Kotloff RM, Rosengard BR, Arcasoy SM, Blumenthal NP, Kaiser LR, et al. Bilateral versus single lung transplantation for chronic obstructive pulmonary disease: intermediate-term results. Ann Thorac Surg. 2000;70(6):1813-8. https://doi.org/10.1016/S0003-4975(00)01970-6
5. Morlion B, Knoop C, Paiva M, Estenne M. Internet-based home monitoring of pul-monary function after lung transplantation. Am J Respir Crit Care Med. 2002;165(5):694-7. https://doi.org/10.1164/ajrccm.165.5.2107059




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Pocket
Pocket