ABSTRACT
Objective: Multidrug-resistant tuberculosis (MDR-TB) remains a global public health challenge, complicating treatment strategies and requiring advanced therapeutic approaches. The persistence of MDR-TB has led to a demand for regimens that are more effective in improving treatment outcomes and controlling transmission. This systematic review and meta-analysis sought to examine the efficacy of linezolid (LZD) and bedaquiline (BDQ) in MDR-TB treatment regimens, evaluating their roles in enhancing therapeutic success and informing optimized management of MDR-TB. Methods: A comprehensive search was conducted across MEDLINE (PubMed), EMBASE, the Cochrane Central Register of Controlled Trials, Scopus, and Web of Science for randomized controlled trials assessing the efficacy of LZD and BDQ in MDR-TB patients up to September 14, 2024. We analyzed treatment outcomes, reporting favorable outcomes (cured and treatment completed) and unfavorable outcomes (death, treatment failure, and loss to follow-up) with a 95% confidence interval. Results: Our analysis included 11 trials, with a total of 1,999 participants. The findings indicate that BDQ+LZD-containing regimens yield significantly higher favorable treatment outcomes (84.5%; 95% CI, 79.8%-88.2%) and lower unfavorable outcomes (15.4%; 95% CI, 11.6%-20.2%). In contrast, regimens lacking either LZD or BDQ show lower efficacy, with favorable outcomes at 66.8% (95% CI, 59.5%-73.4%) and unfavorable outcomes at 33.0% (95% CI, 25.6%-41.4%). Conclusions: MDR-TB treatment regimens including BDQ and LZD lead to significantly better patient outcomes. The combined bactericidal and protein synthesis-inhibiting effects of BDQ and LZD create a powerful therapeutic synergy. Adding pretomanid further enhances this effectiveness, highlighting its value in complex cases. Future research should focus on optimizing these regimens for safety and efficacy and explore adjunctive therapies to improve MDR-TB outcomes even further.
Keywords:
Linezolid; Tuberculosis; Tuberculosis, multidrug-resistant; Treatment outcome; Systematic review.
INTRODUCTION Multidrug-resistant tuberculosis (MDR-TB) represents a significant public health threat. The treatment of MDR-TB needs either prolonged regimens involving multiple antibiotics or shorter regimens that include newer and more expensive (or difficult-to-obtain) drugs. This situation may create considerable challenges for health care systems, especially in low- and middle-income countries, where resources are limited.(1-6) The rising incidence of drug-resistant tuberculosis (DR-TB) not only leads to longer and more costly treatments but also exacerbates health disparities, raising urgent concerns for global health and economic stability.(7) As health care systems grapple with the dual burden of rising MDR-TB cases and limited resources, the need for effective and accessible treatment options has never been more critical.
In the last 15 years, the role of linezolid (LZD) and bedaquiline (BDQ) as cornerstones of MDR-TB treatment has emerged, and much has been studied on their safety and efficacy.(8-18) These studies have explored the efficacy of regimens including LZD or BDQ, highlighting their potential to improve treatment outcomes and reduce mortality rates.
In response to the MDR-TB crisis, the WHO revised treatment guidelines in 2022 to recommend combinations of BDQ, LZD, and pretomanid (Pa), with or without moxifloxacin: the all-oral six-month BPaL and BPaLM regimens.(19) A further revision occurred in 2024, and the regimens employed in recent clinical trials were recommended as well.(4) These new treatment protocols always include both drugs (i.e., LZD and BDQ), the goal being to enhance therapeutic outcomes and minimize the economic impact on health care systems. Nevertheless, to our knowledge, no previous systematic review and meta-analysis has investigated the combined role of these two core WHO group A drugs.(20-23) Furthermore, the societal implications of these advancements extend beyond clinical efficacy; they encompass economic factors, access to care, and the broader impact of antimicrobial resistance on public health.
The objective of this systematic review and meta-analysis was to examine the efficacy of LZD and BDQ in MDR-TB treatment regimens, evaluating their roles in enhancing therapeutic success and informing optimized management of MDR-TB.
METHODS Definitions MDR-TB is characterized as a variant of tuberculosis induced by Mycobacterium tuberculosis strains that exhibit resistance to at least two fundamental antituberculosis agents: isoniazid and rifampin. The classification of extensively DR-TB (XDR-TB) has undergone significant refinement over time. Initially, XDR-TB was defined as tuberculosis resulting from MDR-TB strains with additional resistance to any fluoroquinolone and at least one of the three second-line injectable agents: kanamycin, amikacin, or capreomycin.(24,25) The 2021 WHO definition of XDR-TB now describes resistance to group A MDR-TB drugs, which include FLQs, LZD, and BDQ.(26)
Prior to 2021, pre-XDR-TB was informally characterized as MDR-TB exhibiting additional resistance to either fluoroquinolones or second-line injectable agents. However, the WHO has revised the definition of XDR-TB to specify that it must include resistance to a fluoroquinolone and either LZD or BDQ, thereby requiring resistance to two of the three group A drugs.(25-28)
Search strategy We conducted a comprehensive literature search across five major databases—MEDLINE (PubMed), EMBASE, the Cochrane Central Register of Controlled Trials, Scopus, and Web of Science—from January 1, 2009 to September 14, 2024 to identify randomized controlled trials assessing the efficacy of BDQ and LZD and treatment outcomes in DR-TB. The search employed the following search terms in each database separately: “Tuberculosis,” “mycobacterium tuberculosis,” “TB,” “MTB,” “tuberculosis,” “Multi-drug resistant,” “multi drug resistant,” “multi drug-resistant,” “multidrug resistant,” “multi-drug resistance,” “multi drug resistance,” “multi drug-resistance,” “multidrug resistance,” “MDR,” “MDR-TB,” “extensively drug resistant,” “extensively drug-resistant,” “extensively drug resistance,” “extensively drug-resistance,” “extensive drug resistant,” “extensive-drug resistant,” “extensive drug-resistant,” “XDR,” “XDR-TB,” “Pre-XDR,” “Pre-XDR-TB,” “pre-XDR TB,” “Rifampicin Resistant,” “outcome.”
This study was conducted and reported by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement(29) and was registered with the International Prospective Register of Systematic Reviews (Identifier: CRD42024603453).
Study selection All collected records were consolidated, and duplicates were eliminated with the use of EndNote X8 (Thomson Reuters, Toronto, ON, Canada). Two reviewers independently screened the titles and abstracts, with disagreements being resolved by a third reviewer. They then assessed the full texts of all potentially eligible studies, and any remaining discrepancies were resolved by the third reviewer.
Eligible studies were selected on the basis of the Population, Intervention, Comparator, and Outcome framework, as follows:
- study design—randomized and nonrandomized controlled trials examining the efficacy of LZD and BDQ in patients with DR-TB
- population—patients ≥ 14 years of age with confirmed DR-TB, including rifampin-resistant tuberculosis, MDR-TB, pre-XDR-TB, and XDR-TB
- intervention—treatment regimens including LZD, BDQ, or both as part of the therapeutic approach to DR-TB
- comparator—comparator arms receiving regimens without LZD and BDQ
- outcome—measured outcomes included treatment success rates, culture conversion, mortality, loss to follow-up, and treatment failure
Articles were excluded if they were cohort studies, case-control studies, cross-sectional studies, case reports/series, reviews, editorials, or conference abstracts. Studies that lacked sufficient data on resistance to LZD and BDQ in DR-TB isolates were also excluded, as were those focusing solely on pregnant women. Additionally, studies not reporting treatment outcomes or using outcomes inconsistent with WHO definitions were omitted.
Data extraction Two authors systematically extracted data into a predefined Microsoft Excel spreadsheet (Microsoft, Redmond, WA, USA). Any discrepancies were resolved with a third reviewer. The extracted data included parameters such as the first author; publication year; study design; study period; country and setting; patient demographics (including age, male count, BMI, prevalence of diabetes mellitus, tobacco use, HIV status, and clinical forms); treatment outcome definitions; number of DR-TB cases; and treatment outcomes. A successful outcome was defined as the sum of “cured” and “treatment completed,” whereas an unsuccessful outcome included “treatment failure,” “loss to follow-up,” and “death.”
Quality assessment The quality of the studies was evaluated by two reviewers using distinct assessment tools, with a third reviewer resolving any inconsistencies. For experimental studies, the Cochrane tool was employed, which assesses various criteria, including random sequence generation, allocation concealment, participant and personnel blinding, outcome assessor blinding, completeness of outcome data, and considerations for selective reporting and other biases. Each study was classified on the basis of the risk of bias: a low risk indicated no concerns; a high risk indicated concerns; and an unclear risk was assigned when information was lacking.
Data analysis Statistical computations were performed with the Comprehensive Meta-Analysis software, version 3.0 (Biostat, Inc., Englewood, NJ, USA). We calculated pooled estimates and 95% confidence intervals for the proportion of patients achieving treatment outcomes. The choice between a random-effects or fixed-effects model was determined by the heterogeneity of effect sizes, as assessed by Cochran’s Q test and the I2 statistic. Additionally, publication bias was evaluated by Begg’s test, with a value of p < 0.05 being considered statistically significant.
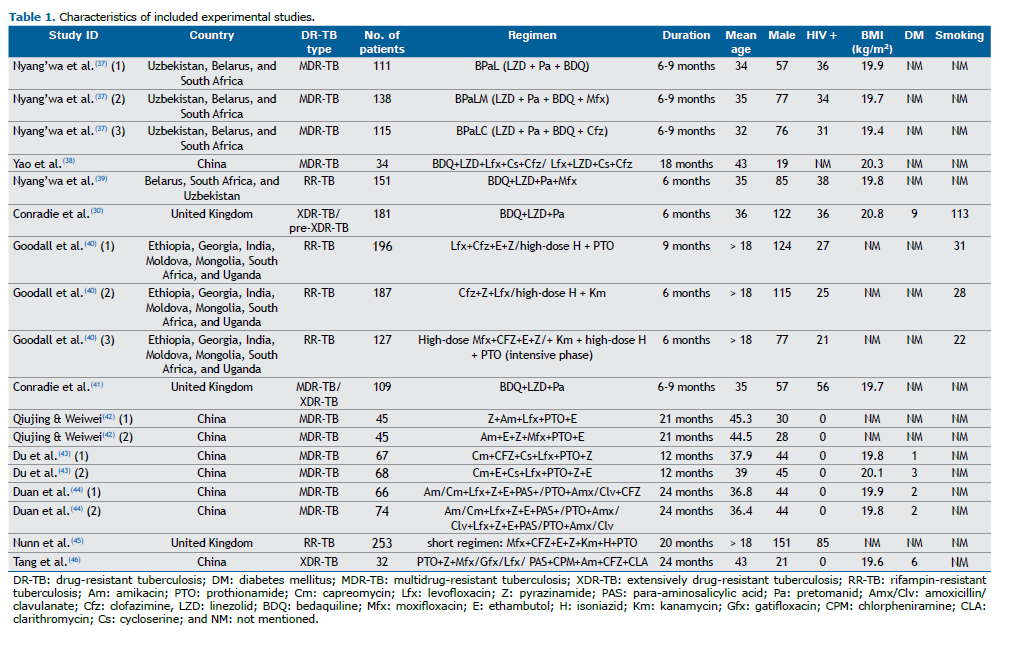
RESULTS As shown in Figure 1, our initial database search identified 8,735 studies. After removing duplicates and conducting title/abstract and full-text screenings, we excluded 8,724 studies, a total of 11 trials including 1,999 patients with various types of DR-TB therefore being included in the final evaluation. All included studies used the previous definition of XDR-TB. The included studies originated from several countries, including India, China, South Korea, and various African nations. The main characteristics of the included studies are shown in Table 1. The mean age of participants was 36.5 years (IQR, 17-71 years). The male-to-female ratio was 1.55, and approximately 19.45% of participants were HIV-positive. Participants were divided into two analytic groups: 839 patients received regimens containing BDQ and LZD, whereas 1,160 patients used regimens that did not include BDQ or LZD. The duration of the studies ranged from 6 months to 24 months, with 1,748 individuals being classified as having rifampin-resistant tuberculosis/MDR-TB and 251 individuals being classified as having pre-XDR-TB/XDR-TB.
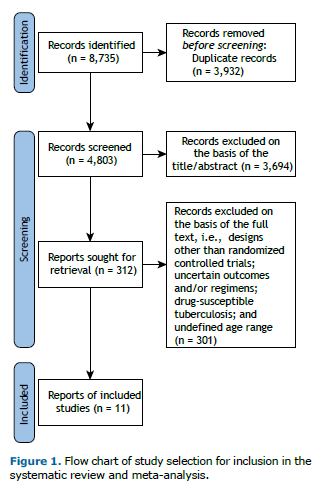
In regimens that included both BDQ and LZD, Pa was the most frequently used drug, often accompanied by moxifloxacin and clofazimine. In contrast, non-BDQ/LZD regimens commonly featured levofloxacin, clofazimine, and ethambutol, along with additional combinations that included amikacin and capreomycin. These non-BDQ/LZD regimens generally exhibited varying levels of efficacy, typically resulting in higher rates of unfavorable outcomes in comparison with BDQ-LZD combinations.
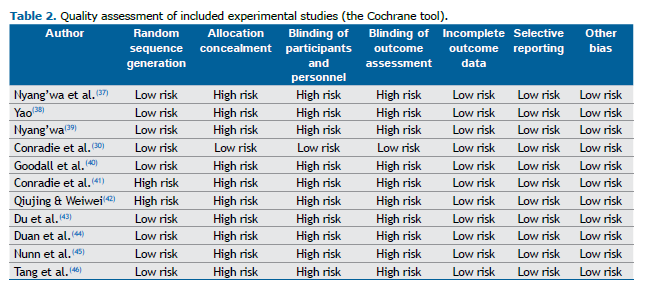
Quality of the included studies The quality of the included clinical trials was assessed with the Cochrane tool. The checklist showed that the included studies had a low risk of bias (Table 2). Of the included studies, the one conducted by Conradie et al.(30) showed a high risk of blinding of participants, personnel, and outcome assessment.
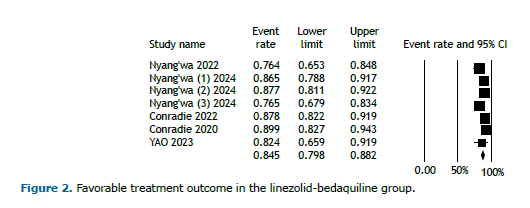
Pooled treatment outcomes in the BDQ-LZD group Five studies featured regimens that included both BDQ and LZD. In the cohort of 839 patients receiving the BDQ-LZD regimen, 645 achieved favorable outcomes, whereas 114 experienced unfavorable outcomes. Favorable outcomes were classified as either cured or treatment completed, whereas unfavorable outcomes included treatment failure, death, and loss to follow-up.
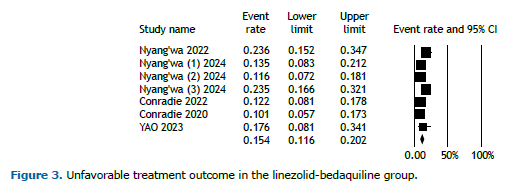
Figure 2 displays the analysis of favorable outcomes among participants receiving the BDQ-LZD regimen, demonstrating an overall favorable outcome rate of 84.5% (95% CI, 79.8%-88.2%). In contrast, the overall unfavorable outcomes for patients on this regimen were reported at 15.4% (95% CI, 11.6%-20.2%; Figure 3). Because of the limited duration of the trials, no temporal trend was observed.
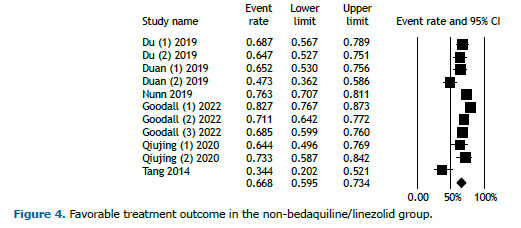
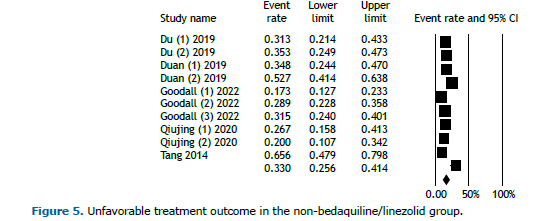
Pooled treatment outcomes in the non-BDQ/LZD group Six studies involved regimens that did not include BDQ or LZD. Of the 1,160 patients in this group, 816 achieved favorable outcomes. Figure 4 illustrates the analysis of favorable outcomes for individuals receiving regimens without BDQ and LZD, revealing an overall favorable outcome rate of 66.8% (95% CI, 59.5%-73.4%). Conversely, the overall unfavorable outcomes among patients on these regimens were reported at 33.0% (95% CI, 25.6%-41.4%; Figure 5). Because of the limited duration of the trials, no temporal trend was observed.
DISCUSSION The results of this analysis clearly demonstrate that treatment regimens incorporating BDQ and LZD yield significantly better patient outcomes than do those that do not include both agents. The BDQ+LZD-based regimens achieved an impressive favorable outcome rate of 84.5%, whereas the non-BDQ/LZD group had a substantially lower favorable outcome rate of 66.8%. This pronounced difference highlights the superior bactericidal and sterilizing activity of BDQ and LZD, both of which are critical in effectively treating MDR-TB.
One of the key advantages of BDQ and LZD lies in their ability to target different sites of the bacterial cell, resulting in rapid bacterial clearance, reduced risk of resistance, and reduced risk of relapse. BDQ disrupts ATP synthase, essential for M. tuberculosis survival, whereas LZD inhibits protein synthesis, together creating a potent synergistic effect that accelerates treatment response.
The association of BDQ with LZD is not however sufficient for the effective treatment of MDR-TB strains. They need accompanying drugs, such as fluoroquinolones, Pa, and clofazimine. Although it was beyond the scope of this review to investigate the best companions for BDQ and LZD, some considerations can be made.
Although injectables (aminoglycosides) now seem obsolete, fluoroquinolones and Pa appear to play an important role in building a BDQ+LZD-based regimen for MDR-TB treatment. Although fluoroquinolones contribute to the bactericidal activity of non-BDQ/LZD regimens, they show reduced efficacy in comparison with combinations of BDQ and LZD. When Pa is added to BDQ-LZD regimens, it further enhances treatment efficacy by disrupting M. tuberculosis cell wall synthesis and targeting persister cells, which are challenging to eradicate even with standard regimens for drug-susceptible tuberculosis.
Although BDQ, LZD, and Pa are crucial for MDR-TB treatment, their toxicity profiles warrant careful monitoring. BDQ has been associated with QT interval prolongation, which poses a risk of cardiac arrhythmias, particularly in patients with preexisting heart conditions or those on concurrent QT-prolonging drugs.(11,30-32) Although LZD is effective, it is linked to bone marrow suppression, peripheral neuropathy, and optic neuropathy, especially when used for long periods of time. Recent studies have emphasized that monitoring blood counts and neurological symptoms can help mitigate these risks.(33-35) Although Pa has been less studied, it has been associated with hepatotoxicity and gastrointestinal side effects, which are exacerbated in patients with liver conditions. (36) These findings underscore the importance of balancing efficacy with safety through vigilant toxicity monitoring to optimize patient outcomes while minimizing adverse effects.
Our study has some notable limitations. First, the variability in study designs, sample sizes, and treatment protocols across the included trials may introduce heterogeneity, affecting the generalization of our findings. Second, data on patient demographics and comorbidities were sometimes insufficient, limiting our ability to fully evaluate their influence on treatment outcomes. Although we focused on the efficacy/effectiveness of BDQ and LZD, this analysis may overlook the potential synergistic effects of other essential drugs in combination therapies, which could significantly impact patient success. In particular, no study included delamanid in any treatment regimen. Additionally, because of the dynamic nature of MDR-TB treatment guidelines, ongoing research is needed to assess the long-term effectiveness of these regimens in real-world settings.
Interestingly, additional information will be available when the full results of two major clinical trials have been published. These two studies proposed a series of different and unusual but effective BDQ-LZD combinations of drugs without Pa, but with fluoroquinolones and/or delamanid.(6) The results, which are probably stunning, have been disclosed to the WHO, leading to a new recommendation for the treatment of patients with MDR-TB.(4)
In conclusion, the present study demonstrates that treatment regimens incorporating BDQ and LZD offer significantly improved outcomes for MDR-TB patients in comparison with regimens without these agents. The synergy between the bactericidal effects of BDQ and the protein synthesis inhibition by LZD provides a powerful approach to combatting M. tuberculosis, resulting in higher rates of favorable outcomes. The addition of Pa further enhances the effectiveness of BDQ-LZD regimens, reinforcing its value in complex MDR-TB cases. Future research should aim to refine these regimens, balancing safety and efficacy, and explore adjunctive therapies to further improve MDR-TB treatment outcomes.
ACKNOWLEDGEMENTS This study is part of the research activities of the Global Tuberculosis Network (GTN) and was supported by Fondo Ricerca Corrente, Ministry of Health, Italy.
AUTHOR CONTRIBUTIONS All authors contributed equally to the conception and design of the study; the collection, analysis, and interpretation of data; and the writing of the manuscript.
CONFLICTS OF INTEREST None declared.
REFERENCES 1. Migliori GB, Sotgiu G, D’Arcy Richardson M, Centis R, Facchini A, Guenther G, et al. MDR-TB and XDR-TB: drug resistance and treatment outcomes. Eur Respir J. 2009;34(3):778-779. https://doi.org/10.1183/09031936.00059409
2. Motta I, Boeree M, Chesov D, Dheda K, Günther G, Horsburgh CR Jr, et al. Recent advances in the treatment of tuberculosis. Clin Microbiol Infect. 2024;30(9):1107-1114. https://doi.org/10.1016/j.cmi.2023.07.013
3. Wei X, Yue L, Zhao B, Jiang N, Lei H, Zhai X. Recent advances and challenges of revolutionizing drug-resistant tuberculosis treatment. Eur J Med Chem. 2024;277:116785. https://doi.org/10.1016/j.ejmech.2024.116785
4. World Health Organization (WHO) [homepage on the Internet]. Geneva: WHO; c2024 [updated 2024 Aug 19; cited 2024 Nov 1]. Key updates to the treatment of drug-resistant tuberculosis: rapid communication, June 2024. Available from: https://www.who.int/publications/i/item/B09123
5. Saderi L, Cabibbe AM, Puci M, Di Lorenzo B, Centis R, Pontali E, et al. A systematic review of the costs of diagnosis for multidrug-resistant/extensively drug-resistant TB in different settings. Int J Tuberc Lung Dis. 2023;27(5):348-356. https://doi.org/10.5588/ijtld.22.0657
6. Pontali E, Raviglione M. Updated treatment guidelines for drug-resistant TB: how safe are clofazimine-based regimens?. IJTLD Open. 2024;1(11):486-489. https://doi.org/10.5588/ijtldopen.24.0490
7. World Health Organization (WHO) [homepage on the Internet]. Geneva: WHO; c2024 [updated 2024 Oct 29; cited 2024 Nov 1]. Global Tuberculosis Report 2024. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024
8. Sotgiu G, Pontali E, Migliori GB. Linezolid to treat MDR-/XDR-tuberculosis: available evidence and future scenarios. Eur Respir J. 2015;45(1):25-29. https://doi.org/10.1183/09031936.00145014
9. Borisov SE, Dheda K, Enwerem M, Romero Leyet R, D’Ambrosio L, Centis R, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017;49(5):1700387. https://doi.org/10.1183/13993003.00387-2017
10. Borisov S, Danila E, Maryandyshev A, Dalcolmo M, Miliauskas S, Kuksa L, et al. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: first global report. Eur Respir J, 2019. 54(6).
11. Pontali E, Sotgiu G, Tiberi S, D’Ambrosio L, Centis R, Migliori GB. Cardiac safety of bedaquiline: a systematic and critical analysis of the evidence. Eur Respir J. 2017;50(5):1701462. https://doi.org/10.1183/13993003.01462-2017
12. Koirala S, Borisov S, Danila E, Mariandyshev A, Shrestha B, Lukhele N, et al. Outcome of treatment of MDR-TB or drug-resistant patients treated with bedaquiline and delamanid: Results from a large global cohort. Pulmonology, 2021;27(5):403-412. https://doi.org/10.1016/j.pulmoe.2021.02.006
13. Borisov SE, D’Ambrosio L, Centis R, Tiberi S, Dheda K, Alffenaar JW, et al. Outcomes of patients with drug-resistant-tuberculosis treated with bedaquiline-containing regimens and undergoing adjunctive surgery. J Infect. 2019;78(1):35-39. https://doi.org/10.1016/j.jinf.2018.08.003
14. Pontali E, Sotgiu G, Tiberi S, Tadolini M, Visca D, D’Ambrosio L, et al. Combined treatment of drug-resistant tuberculosis with bedaquiline and delamanid: a systematic review. Eur Respir J. 2018;52(1):1800934. https://doi.org/10.1183/13993003.00934-2018
15. Sotgiu G, Centis R, D’Ambrosio L, Alffenaar JW, Anger HA, Caminero JA, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J. 2012;40(6):1430-42. https://doi.org/10.1183/09031936.00022912
16. Oehadian A, Bastos ML, Centis R, D’Ambrosio L, Migliori GB, Santoso P, et al. Occurrence and predictors of adverse events associated with Linezolid in the treatment of patients with MDR-TB. Pulmonology. 2024;30(2):184-187. https://doi.org/10.1016/j.pulmoe.2023.09.003
17. Bolhuis MS, Akkerman OW, Sturkenboom MGG, Ghimire S, Srivastava S, Gumbo T, et al. Linezolid-based Regimens for Multidrug-resistant Tuberculosis (TB): A Systematic Review to Establish or Revise the Current Recommended Dose for TB Treatment. Clin Infect Dis. 2018;67(suppl_3):S327-S335. https://doi.org/10.1093/cid/ciy625
18. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017; Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JC, Anderson LF, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821-834. https://doi.org/10.1016/S0140-6736(18)31644-1
19. World Health Organization (WHO) [homepage on the Internet]. Geneva: WHO; c2023 [cited 2024 Nov 1].Global tuberculosis report 2023. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023
20. Hatami H, Sotgiu G, Bostanghadiri N, Abadi SSD, Mesgarpour B, Goudarzi H, et al. Bedaquiline-containing regimens and multidrug-resistant tuberculosis: a systematic review and meta-analysis. J Bras Pneumol. 2022;48(2):e20210384. https://doi.org/10.36416/1806-3756/e20210384
21. Koirala S, Borisov S, Danila E, Mariandyshev A, Shrestha B, Lukhele N, et al. Outcome of treatment of MDR-TB or drug-resistant patients treated with bedaquiline and delamanid: Results from a large global cohort. Pulmonology. 2021;27(5):403-412. https://doi.org/10.1016/j.pulmoe.2021.02.006
22. Padmapriyadarsini C, Vohra V, Bhatnagar A, Solanki R, Sridhar R, Anande L, et al. Bedaquiline, delamanid, linezolid, and clofazimine for treatment of pre-extensively drug-resistant tuberculosis. Clin Infect Dis. 2022;76(3):e938-46. https://doi.org/10.1093/cid/ciac528
23. Hatami H, Sotgiu G, Bostanghadiri N, Abadi SSD, Mesgarpour B, Goudarzi H, et al. Bedaquiline-containing regimens and multidrug-resistant tuberculosis: a systematic review and meta-analysis. J Bras Pneumol. 2022;48(2):e20210384. https://doi.org/10.36416/1806-3756/e20210384
24. World Health Organization, Guidelines for surveillance of drug resistance in tuberculosis, 4th ed. Geneva: World Health Organization; 2009.
25. Borisov S, Danila E, Maryandyshev A, Dalcolmo M, Miliauskas S, Kuksa L, et al. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: first global report. Eur Respir J. 2019;54(6):1901522. https://doi.org/10.1183/13993003.01522-2019
26. Viney K, Linh NN, Gegia M, Zignol M, Glaziou P, Ismail N, et al. New definitions of pre-extensively and extensively drug-resistant tuberculosis: update from the World Health Organization. Eur Respir J. 2021;57(4):2100361. https://doi.org/10.1183/13993003.congress-2021.OA1599
27. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017; Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JC, Anderson LF, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821-834. https://doi.org/10.1016/S0140-6736(18)31644-1
28. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment-drug-resistant tuberculosis treatment, 2022 update. Geneva: WHO; 2022.
29. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
30. Conradie F, Bagdasaryan TR, Borisov S, Howell P, Mikiashvili L, Ngubane N, et al. Bedaquiline-Pretomanid-Linezolid Regimens for Drug-Resistant Tuberculosis. N Engl J Med. 2022;387(9):810-823. https://doi.org/10.1056/NEJMoa2119430
31. Guglielmetti L, Tiberi S, Burman M, Kunst H, Wejse C, Togonidze T, et al. QT prolongation and cardiac toxicity of new tuberculosis drugs in Europe: a Tuberculosis Network European Trialsgroup (TBnet) study. Eur Respir J. 2018;52(2):1800537. https://doi.org/10.1183/13993003.00537-2018
32. Motta I, Cozzi SN, Pontali E. QT prolongation for old and new drugs: how much should we really worry?. Int J Tuberc Lung Dis. 2022;26(4):298-301. https://doi.org/10.5588/ijtld.22.0072
33. Veerman K, Goosen J, Spijkers K, Jager N, Heesterbeek P, Telgt D. Prolonged use of linezolid in bone and joint infections: a retrospective analysis of adverse effects. J Antimicrob Chemother. 2023;78(11):2660-2666. https://doi.org/10.1093/jac/dkad276
34. Hasan T, Medcalf E, Nyang’wa BT, Egizi E, Berry C, Dodd M, et al. The Safety and Tolerability of Linezolid in Novel Short-Course Regimens Containing Bedaquiline, Pretomanid, and Linezolid to Treat Rifampicin-Resistant Tuberculosis: An Individual Patient Data Meta-analysis. Clin Infect Dis. 2024;78(3):730-741. https://doi.org/10.1093/cid/ciad653
35. Wasserman S, Brust JCM, Abdelwahab MT, Little F, Denti P, Wiesner L, et al. Linezolid toxicity in patients with drug-resistant tuberculosis: a prospective cohort study. J Antimicrob Chemother. 2022;77(4):1146-1154. https://doi.org/10.1093/jac/dkac019
36. Ramachandran G, Swaminathan S. Safety and tolerability profile of second-line anti-tuberculosis medications. Drug Saf. 2015;38(3):253-269. https://doi.org/10.1007/s40264-015-0267-y
37. Nyang’wa BT, Berry C, Kazounis E, Motta I, Parpieva N, Tigay Z, et al. Short oral regimens for pulmonary rifampicin-resistant tuberculosis (TB-PRACTECAL): an open-label, randomised, controlled, phase 2B-3, multi-arm, multicentre, non-inferiority trial. Lancet Respir Med. 2024;12(2):117-128. https://doi.org/10.1016/S2213-2600(23)00389-2
38. Yao G, Zhu M, Nie Q, Chen N, Tu S, Zhou Y, et al. Improved outcomes following addition of bedaquiline and clofazimine to a treatment regimen for multidrug-resistant tuberculosis. J Int Med Res. 2023;51(1):3000605221148416. https://doi.org/10.1177/03000605221148416
39. Nyang’wa BT, Berry C, Kazounis E, Motta I, Parpieva N, Tigay Z, et al. A 24-Week, All-Oral Regimen for Rifampin-Resistant Tuberculosis. N Engl J Med. 2022;387(25):2331-2343. https://doi.org/10.1056/NEJMoa2117166
40. Goodall RL, Meredith SK, Nunn AJ, Bayissa A, Bhatnagar AK, Bronson G, et al. Evaluation of two short standardised regimens for the treatment of rifampicin-resistant tuberculosis (STREAM stage 2): an open-label, multicentre, randomised, non-inferiority trial. [published correction appears in Lancet. 2022 Nov 19;400(10365):1766. doi: 10.1016/S0140-6736(22)02307-8]. Lancet. 2022;400(10366):1858-1868. https://doi.org/10.1016/S0140-6736(22)02078-5
41. Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N Engl J Med. 2020;382(10):893-902. https://doi.org/10.1056/NEJMoa1901814
42. Qiujing F, Weiwei W. Efficacy of moxifloxacin combined with levofloxacin in the treatment of drug-resistant tuberculosis. Pak J Pharm Sci. 2020;33(3(Special)):1361-1366. PMID: 33361023.
43. Du Y, Qiu C, Chen X, Wang J, Jing W, Pan H, et al. Treatment Outcome of a Shorter Regimen Containing Clofazimine for Multidrug-resistant Tuberculosis: A Randomized Control Trial in China. Clin Infect Dis. 2020;71(4):1047-1054. https://doi.org/10.1093/cid/ciz915
44. Duan H, Chen X, Li Z, Pang Y, Jing W, Liu P, et al. Clofazimine improves clinical outcomes in multidrug-resistant tuberculosis: a randomized controlled trial. Clin Microbiol Infect. 2019;25(2):190-195. https://doi.org/10.1016/j.cmi.2018.07.012
45. Nunn AJ, Phillips PPJ, Meredith SK, Chiang CY, Conradie F, Dalai D, et al. A Trial of a Shorter Regimen for Rifampin-Resistant Tuberculosis. N Engl J Med. 2019;380(13):1201-1213. https://doi.org/10.1056/NEJMoa1811867
46. Tang S, Yao L, Hao X, Zhang X, Liu G, Liu X, et al., Efficacy, safety and toler-ability of linezolid for the treatment of XDR-TB: a study in China. Eur Respir J. 2015;45(1):161-70. https://doi.org/10.1183/09031936.00035114




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Pocket
Pocket