BACKGROUND The number of elderly individuals (≥ 65 years of age) worldwide is projected to triplicate by 2050, with a quarter of these individuals being in the “oldest-old” age range (> 85 years of age).(1) The prevalence of chronic lung disease and comorbidities with the potential to influence pulmonary function tests increases with aging. Knowing the physiological effects of senescence on the respiratory system is paramount to avoiding under- or overdiagnosis of respiratory disease in the elderly.
OVERVIEW A 77-year-old man with a smoking history of 50 pack-years—he had quit 10 years before—heart failure (left ventricular ejection fraction = 36%), and atrial fibrillation presented with progressive dyspnea (modified Medical Research Council scale score = 3) after a lower respiratory tract infection that was managed at home. A chest X-ray showed minor linear opacities in the right lower lobe. He was diagnosed with COPD on the basis of the following: a) FEV1/FVC < 0.7 (but above the lower limit of normal); b) FEV1/”slow” VC below the lower limit of normal; c) a borderline decrease in FEF25-75%, with some expiratory “scooping”; d) mildly increased RV; and e) slightly decreased DLCO. Inhaled formoterol did not improve his dyspnea, being associated with palpitations and lightheadedness. Because of these undesirable side effects, formoterol was discontinued. His dyspnea eventually subsided after a few weeks of chest physiotherapy for secretion clearance.
Aging is associated with loss of lung elastic recoil and alveolar attachments to the small airways, both of which contribute to decreasing the expiratory flows and, consequently, FEV1 and FEF25-75% (a-c above). Low mid- and tele-expiratory flow may create a slight concavity on the expiratory flow limb (c). An increase in the relaxation volume of the respiratory system and a tendency toward airway closure at a small lung volume cause an increase in functional residual capacity and RV (d), respectively. TLC may remain unchanged or decrease secondary to a stiffer chest wall, reducing inspiratory capacity and VC.(2) Given that the small airways tend to close earlier during a forced expiratory maneuver than during a “slow” expiratory maneuver, FVC decreases more than does VC; thus, FEV1/VC diminishes to a greater extent than does FEV1/FVC (a and b above).(3) Since the volume at which the small airways start to close during expiration increases more than does functional residual capacity, ventilation distribution inequalities may decrease pulmonary gas exchange efficiency. Airspace dilation without distinct alveolar destruction and reduced density of the membranous bronchioles suggest coalescence of smaller alveoli into larger alveoli, reducing the functional surface for gas exchange while increasing areas of a high ventilation-perfusion relationship.(2) The corollary is an age-related reduction in DLCO (e) and PaO2, as well as an increase in the alveolar-arterial oxygen gradient (Table 1).
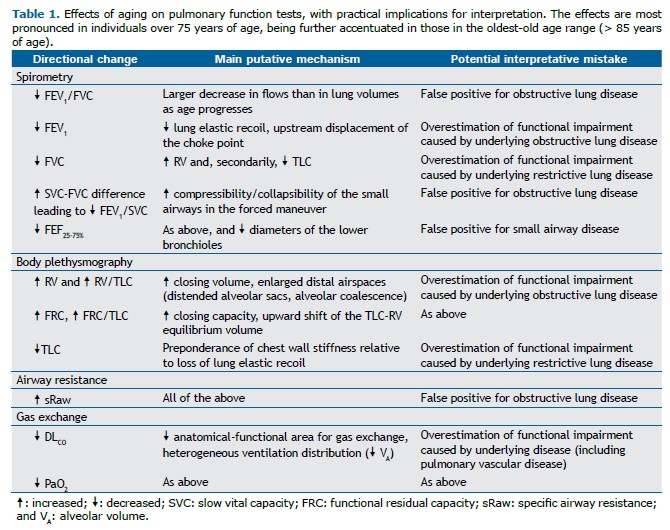
Several aging-related physiological changes can mimic the abnormalities induced by airway disease, including low expiratory flows, increased operating lung volumes, and ventilation distribution inequalities. Conversely, the dominance of chest wall stiffness relative to the loss of lung elastic recoil may raise unjustified concerns of restriction, particularly in the presence of moderate-to-severe obesity.(4) This scenario is further complicated by the lower accuracy of reference values in the extremes of age.(5) Special care should be taken to avoid overdiagnosis of respiratory disease (or overestimation of impairment caused by preexisting disease) in the elderly.
REFERENCES 1. National Institutes of Health. National Institute on Aging [homepage on the Internet]; Bethesda: National Institute on Aging [updated: 2011 Oct; cited: 2022 Jul 19]. Global health and aging: Preface. [Adobe Acrobat document; 32p.]. Available from: https://www.nia.nih.gov/sites/default/files/2017-06/global_health_aging.pdf
2. Neder JA, Berton DC, O’Donnell DE. The Lung Function Laboratory to Assist Clinical Decision-making in Pulmonology: Evolving Challenges to an Old Issue. Chest. 2020;158(4):1629-1643. https://doi.org/10.1016/j.chest.2020.04.064
3. Saint-Pierre M, Ladha J, Berton DC, Reimao G, Castelli G, Marillier M,et al. Is the Slow Vital Capacity Clinically Useful to Uncover Airflow Limitation in Subjects With Preserved FEV1/FVC Ratio?. Chest. 2019;156(3):497-506. https://doi.org/10.1016/j.chest.2019.02.001
4. Marillier M, Bernard AC, Reimao G, Castelli G, Alqurashi H, O’Donnell DE, et al. Breathing at Extremes: The Restrictive Consequences of Super- and Super-Super Obesity in Men and Women. Chest. 2020;158(4):1576-1585. https://doi.org/10.1016/j.chest.2020.04.006
5. Neder JA, Berton DC, O’Donnell DE. Lung function: what constitutes (ab)normality?. J Bras Pneumol. 2022;48(2):e20220096. https://doi.org/10.36416/1806-3756/e20220096


