ABSTRACT
Objective: To determine whether abnormal continuous glucose monitoring (CGM) readings (hypoglycemia/hyperglycemia) can predict the onset of cystic fibrosis-related diabetes (CFRD) and/or clinical impairment (decline in BMI and/or FEV1) in pediatric patients with cystic fibrosis (CF). Methods: This was a longitudinal prospective cohort study involving CF patients without diabetes at baseline. The mean follow-up period was 3.1 years. The patients underwent 3-day CGM, performed oral glucose tolerance test (OGTT), and had FEV1 and BMI determined at baseline. OGTT, FEV1, and BMI were reassessed at the end of the follow-up period. Results: Thirty-nine CF patients (10-19 years of age) had valid CGM readings at baseline, and 34 completed the follow-up period (mean = 3.1 ± 0.5 years). None of the study variables predicted progression to CFRD or were associated with hypoglycemic events. CGM could detect glucose abnormalities not revealed by OGTT. Patients with glucose levels = 140 mg/dL, as compared with those with lower levels, on CGM showed lower BMI values and z-scores at baseline-17.30 ± 3.91 kg/m2 vs. 19.42 ± 2.07 kg/m2; p = 0.043; and -1.55 ± 1.68 vs. -0.17 ± 0.88; p = 0.02, respectively-and at the end of follow-up-17.88 ± 3.63 kg/m2 vs. 19.95 ± 2.56 kg/m2; p = 0.039; and -1.65 ± 1.55 vs. -0.42 ± 1.08; p = 0.039. When comparing patients with and without CFRD, the former were found to have worse FEV1 (in % of predicted)-22.67 ± 5.03 vs. 59.58 ± 28.92; p = 0.041-and a greater decline in FEV1 (-36.00 ± 23.52 vs. -8.13 ± 17.18; p = 0.041) at the end of follow-up. Conclusions: CGM was able to identify glucose abnormalities not detected by OGTT that were related to early-stage decreases in BMI. CGM was ineffective in predicting the onset of diabetes in this CF population. Different diagnostic criteria for diabetes may be required for individuals with CF.
Keywords:
Cystic fibrosis; Glucose intolerance; Glucose tolerance test; Diabetes mellitus.
RESUMO
Objetivo: Verificar se leituras de continuous glucose monitoring (CGM, monitoramento contínuo da glicose) anormais (hipoglicemia/hiperglicemia) podem prever o aparecimento de diabetes relacionado à fibrose cística (DRFC) e/ou comprometimento clínico (declínio do IMC e/ou do VEF1) em pacientes pediátricos com fibrose cística (FC). Métodos: Estudo de coorte longitudinal prospectivo envolvendo pacientes com FC sem diabetes no início do estudo. O tempo médio de acompanhamento foi de 3,1 anos. Os pacientes foram submetidos a CGM de três dias, teste oral de tolerância à glicose (TOTG) e medida de VEF1 e IMC no início do estudo. TOTG, VEF1 e IMC foram reavaliados ao final do acompanhamento. Resultados: Trinta e nove pacientes com FC (10-19 anos de idade) apresentaram leituras de CGM válidas no início do estudo, e 34 completaram o acompanhamento (média = 3,1 ± 0,5 anos). Nenhuma das variáveis estudadas previu evolução para DRFC ou apresentou associação com eventos hipoglicêmicos. O CGM conseguiu detectar anormalidades glicêmicas não reveladas pelo TOTG. Pacientes com níveis de glicose ≥ 140 mg/dL no CGM, comparados àqueles com níveis menores, apresentaram valores de IMC e escores z de IMC menores no início do estudo — 17,30 ± 3,91 kg/m2 vs. 19,42 ± 2,07 kg/m2; p = 0,043; e −1,55 ± 1,68 vs. −0,17 ± 0,88; p = 0,02, respectivamente — e no final do acompanhamento — 17,88 ± 3,63 kg/m2 vs. 19,95 ± 2,56 kg/m2; p = 0,039; e −1,65 ± 1,55 vs. −0,42 ± 1,08; p = 0,039. Na comparação dos pacientes com e sem DRFC, os primeiros apresentaram pior VEF1 (em % do previsto) — 22,67 ± 5,03 vs. 59,58 ± 28,92; p = 0,041 — e maior declínio do VEF1 (−36,00 ± 23,52 vs. −8,13 ± 17,18; p = 0,041) no final do acompanhamento. Conclusões: O CGM foi capaz de identificar anormalidades glicêmicas não detectadas pelo TOTG que se relacionaram com reduções precoces do IMC. O CGM foi ineficaz na previsão do aparecimento de diabetes nesta população com FC. Diferentes critérios diagnósticos para diabetes podem ser necessários para indivíduos com FC.
Palavras-chave:
Fibrose cística; Intolerância à glucose; Teste de tolerância a glucose; Diabetes mellitus.
INTRODUÇÃO O diabetes relacionado à fibrose cística (DRFC) é a comorbidade mais comum na fibrose cística (FC). Teoriza-se que a fisiopatologia do DRFC envolva insuficiência de insulina, mas, diferentemente do diabetes mellitus tipo 1, o dano às células β na FC não é causado por autoimunidade e está associado a algum grau de resistência à insulina causada por inflamação e medicamentos.(1)
O DRFC correlaciona-se com declínio progressivo da função pulmonar e do estado nutricional e, portanto, menor sobrevida.(2,3) Segundo recomendações de uma publicação com diretrizes de consenso,(4) o padrão ouro para o rastreamento do DRFC é o teste oral de tolerância à glicose (TOTG). O TOTG é um exame oneroso, pois as amostras são coletadas durante um longo período, é necessário jejum antes do teste, e a baixa tolerabilidade gastrointestinal impõe desafios à adesão.(5) Os TOTGs podem induzir episódios hipoglicêmicos após a carga de glicose.(6-8) O continuous glucose monitoring (CGM, monitoramento contínuo da glicose) pode ser um método sensível para detectar hipoglicemia/hiperglicemia espontânea em pacientes com FC, tendo sido validado para uso em crianças e adolescentes com FC.(9,10)
A hipoglicemia na FC pode estar associada a um atraso na primeira fase de secreção de insulina combinado com uma resposta diminuída de glucagon, doença hepática, desnutrição, distúrbios gastrointestinais e outras disfunções incretínicas. O DRFC e a hipoglicemia na FC compartilham uma base fisiopatológica semelhante.(11) Para o CGM, os valores de corte para hipoglicemia são classificados em dois níveis aplicáveis ao diabetes tipo 1 e tipo 2 (em mg/dL): < 70 (nível 1) e < 54 (nível 2).(12)
Embora exista o risco de complicações microvasculares, o principal objetivo do manejo do DRFC é controlar o crescimento bacteriano pulmonar, evitar o declínio da função pulmonar e do estado nutricional e garantir o controle glicêmico.(13)
O presente estudo teve como objetivo verificar se leituras de CGM anormais (hipoglicemia/hiperglicemia), em comparação com o padrão ouro TOTG, poderiam prever o aparecimento de DRFC e/ou comprometimento clínico (declínio do IMC e/ou do VEF1 em porcentagem do previsto) em pacientes pediátricos com FC.
MÉTODOS Pacientes e desenho do estudo Foi realizado um estudo prospectivo unicêntrico entre agosto de 2014 e janeiro de 2019. Todos os pacientes — de 10,0 a 19,9 anos de idade e com duas variantes patogênicas no gene cystic fibrosis transmembrane conductance regulator (CFTR) e/ou com dois resultados de teste de cloreto no suor ≥ 60 mEq/L — atendidos no ambulatório do centro de referência em FC foram convidados a participar (N = 63).
O teste do suor foi realizado por meio da análise iônica quantitativa do suor (iontoforese) após estimulação da pele com pilocarpina.(14) O teste genético foi realizado por meio de sequenciamento genético; o genótipo foi classificado como homozigoto ou heterozigoto para a variante p.Phe508del e por sua gravidade com base nas classes de mutação do CFTR.
Coleta de dados, análise de dados e medidas de desfecho Os pacientes com FC foram acompanhados durante suas consultas clínicas de rotina trimestrais. Dois momentos foram avaliados neste estudo: T0 (início do estudo), momento em que os participantes foram submetidos ao CGM e ao TOTG, e T1 (final do período de acompanhamento), momento da consulta de rotina mais próxima dos primeiros casos recém-diagnosticados de DRFC pelo TOTG na coorte. Para aqueles que não desenvolveram DRFC, o T1 foi determinado como a última consulta antes do término do estudo.
Nenhum(a) participante apresentava exacerbações pulmonares, fazia uso de corticoterapia sistêmica ou estava grávida no momento da coleta de dados. Indivíduos com calibração inadequada de CGM, leituras de CGM realizadas em < 36 h, que não completaram o TOTG ou que foram diagnosticados com diabetes com base nos critérios da American Diabetes Association(15) foram excluídos. Nenhum paciente recebeu nutrição enteral ou terapia com moduladores de CFTR ou foi submetido a transplante pulmonar durante o período de acompanhamento.
Todos os participantes do estudo usaram o sistema CGM Gold® (Medtronic MiniMed, Fridley, MN, EUA) por no mínimo 36 h e até 3 dias no início do período de acompanhamento. O número de picos (≥ 140 mg/dL e ≥ 200 mg/dL) e vales (< 54 mg/dL) foi ajustado para a duração do CGM. Os dados do CGM foram fornecidos pelo programa MiniMed Solutions, versão 1.7a (Medtronic Minimed). Foram avaliados o número de picos ≥ 140 mg/dL e ≥ 200 mg/dL (total e por dia); o número de vales < 54 mg/dL (total e por dia); a porcentagem de tempo em que os valores de glicose intersticial permaneceram < 54 mg/dL, ≥ 140 mg/dL e ≥ 200 mg/dL; a ASC para valores de glicose intersticial < 54 mg/dL, ≥ 140 mg/dL e ≥ 200 mg/dL; e a associação de picos (mg/dL) ≥ 200 mg/dL com valores < 54 mg/dL.
Os pacientes eram continuamente orientados sobre os sinais clínicos de hipoglicemia (fraqueza, tremores, fome, irritabilidade e outros) durante as consultas. Para o estudo, eles foram orientados outra vez quando o dispositivo de CGM foi colocado. Após a retirada do dispositivo, eles responderam a um questionário sobre complicações durante o uso do dispositivo de CGM, no qual foram questionados ativamente sobre hipoglicemia (valores < 70 mg/dL detectados por meio de medidas de glicemia capilar e/ou sinais clínicos de hipoglicemia).
A classificação do CGM foi baseada nos valores de corte do TOTG para normal glucose tolerance (NGT; tolerância normal à glicose; glicose intersticial < 140 mg/dL), impaired glucose tolerance (IGT, intolerância à glicose; glicose intersticial entre 140 e 199 mg/dL) e DRFC (glicose intersticial ≥ 200 mg/dL pelo menos duas vezes). Além disso, duas análises de subgrupo foram realizadas analisando anormalidades glicêmicas (DRFC + IGT) vs. NGT tanto para o CGM quanto para os TOTGs.
O TOTG foi solicitado anualmente conforme as recomendações de diretrizes(4); no entanto, acompanhamos as variáveis do estudo apenas nos dois momentos estudados (T0 e T1), pois a adesão foi inadequada durante o acompanhamento. Com base nos resultados do TOTG, realizado de acordo com o protocolo da OMS(16) e utilizando o método enzimático colorimétrico, classificamos os participantes de acordo com os critérios da American Diabetes Association(15)—NGT: glicemia de jejum < 126 mg/dL ou glicemia < 140 mg/dL aos 120 min; IGT: glicemia de jejum < 126 mg/dL ou glicemia de 140-199 mg/dL aos 120 min; e diabetes: glicemia de jejum ≥ 126 mg/dL ou glicemia ≥ 200 mg/dL aos 120 min (pelo menos duas vezes).
A espirometria foi realizada em conformidade com as normas da American Thoracic Society e da European Respiratory Society.(17) O VEF1 em porcentagem do previsto foi avaliado em T0 e T1.(18)
Dois endocrinologistas pediátricos avaliaram o peso, altura, IMC e estadiamento puberal dos participantes em T0 e T1. O IMC foi apresentado em valores absolutos e escores z com base nos padrões de crescimento infantil da OMS de 2006.(19) O estadiamento puberal foi avaliado utilizando os critérios de Marshall & Tanner.(20,21)
A função pancreática exócrina (insuficiência pancreática [IP] exócrina < 200 µg/g) foi avaliada com base nos níveis de elastase fecal 1 em T0 utilizando o teste da elastase pancreática fecal 1 (ScheBo, Giessen, Alemanha).(22)
O protocolo do estudo foi aprovado pelo Comitê de Ética em Pesquisa da Faculdade de Ciências Médicas, Universidade Estadual de Campinas (Protocolo n. 3.328.215). Todos os participantes ou seus responsáveis legais assinaram um termo de consentimento livre e esclarecido para participação no estudo. Os menores de idade também assinaram um termo de assentimento.
Análise estatística Todas as análises foram realizadas por meio do programa IBM SPSS Statistics, versão 20.0 (IBM Corp., Armonk, NY, EUA). As diferenças foram consideradas estatisticamente significativas quando p bicaudal < 0,05. As variáveis qualitativas foram expressas em frequências absolutas e relativas, e as variáveis quantitativas foram expressas em medianas e valores mínimos e máximos. Os testes de Mann-Whitney e Kruskal-Wallis foram utilizados para comparar dois e três ou mais grupos independentes, respectivamente. Um teste não paramétrico de comparação múltipla foi utilizado para identificar diferenças entre grupos.
As associações com as variáveis qualitativas foram analisadas pelo teste exato de Fisher ou pelo teste de Fisher-Freeman-Halton, conforme apropriado. Para as avaliações pareadas, utilizou-se o teste de McNemar-Bowker e o teste de Wilcoxon.
Foi realizada análise de regressão logística univariada para identificar preditores de DRFC. Os preditores com p < 0,2 na análise univariada foram incluídos na análise multivariada por modelos de equações de estimação generalizadas.
RESULTADOS Dos 63 pacientes recrutados, 13 se recusaram a participar, 1 teve várias exacerbações pulmonares, 2 forneceram leituras de CGM por < 36 h, e 8 foram diagnosticados com DRFC. Portanto, 39 pacientes não diabéticos com FC foram submetidos a um CGM cegado de 3 dias e foram acompanhados por um período médio de 3,1 ± 0,5 anos. Desses, 34 participantes completaram o acompanhamento do estudo (Figura 1). Em T0, não conseguimos saber quem se tornaria diabético, tínhamos apenas a classificação de indivíduo com IGT ou NGT de acordo com os resultados do TOTG. Além disso, todos os pacientes da coorte tinham função pulmonar e estado nutricional comparáveis em T0. Três pacientes foram classificados como tendo DRFC pelo TOTG em T1.
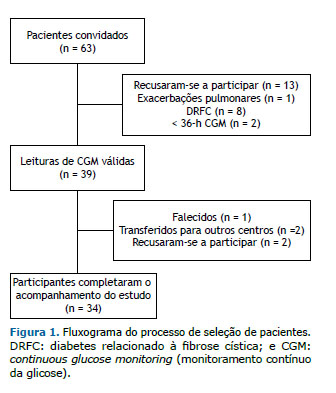
Os dados demográficos são apresentados na Tabela 1. Durante o período de acompanhamento, os pacientes com IP não apresentaran alterações nos parâmetros clínicos, balanço de gordura fecal e esteatócrito. Pacientes com valores de glicemia intersticial < 140 mg/dL (n = 8) no CGM não desenvolveram DRFC durante o período de acompanhamento, e apenas 1 apresentou um único episódio de hipoglicemia assintomática. Todos os pacientes classificados como tendo DRFC com base no CGM apresentaram episódios hipoglicêmicos assintomáticos. Nenhum dos pacientes que evoluíram para DRFC apresentou picos ≥ 200 mg/dL durante o CGM. A relação entre os TOTGs (em T0 e T1) e o CGM é apresentada na Figura 2.
O padrão pico/vale (total e por dia), a ASC e a porcentagem de tempo em que os valores (em mg/dL) foram ≥ 140, ≥ 200 e < 54 no CGM não mostraram associações com a classificação do TOTG nem em T0 nem em T1. As variáveis individuais do CGM estão descritas na Tabela S1.
Onze pacientes (32%) — 7 eram do sexo masculino, 7 eram homozigotos para a variante p.Phe508del do CFTR, e 7 apresentavam IP — tiveram níveis de glicose < 54 mg/dL durante o CGM. Não houve associações do IMC, VEF1, resultados do TOTG, sexo, genótipo p.Phe508del e ID com a hipoglicemia no CGM (em T0). Nenhum dos pacientes que apresentaram hipoglicemia necessitou de intervenção para recuperação. Nenhuma das variáveis clínicas ou laboratoriais analisadas apresentou associação com episódios hipoglicêmicos ou conseguiu prever o aparecimento de DRFC (dados não apresentados).
Uma análise secundária foi realizada agrupando episódios hipoglicêmicos e hiperglicêmicos (≥ 200 mg/dL) para verificar se essa conjunção poderia apresentar relação com o desfecho DRFC; nenhuma associação significativa foi encontrada (p = 0,664).
Os pacientes que desenvolveram DRFC, comparados aos que não desenvolveram a doença, apresentaram pior VEF1 (em % do previsto) — 22,67 ± 5,03 vs. 59,58 ± 28,92; p = 0,041 em T1 (Tabela 2).
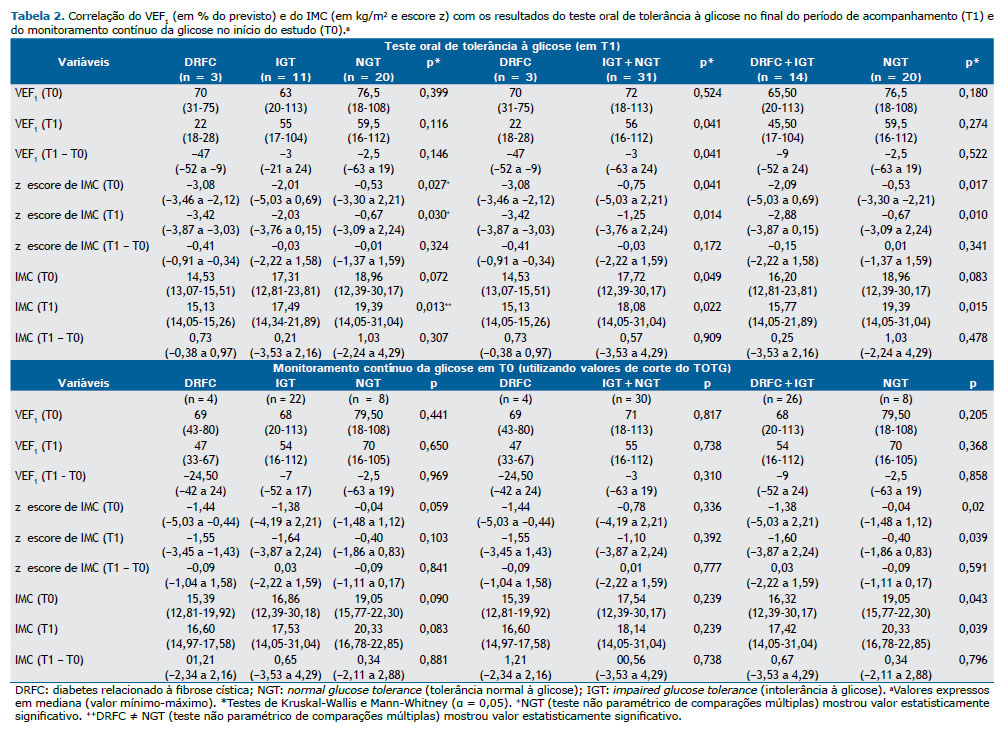
Os escores z de IMC e os valores brutos de IMC são mostrados de acordo com os resultados do TOTG (em T1) e do CGM (em T0) na Tabela 2. Foram observados menores valores de IMC nos pacientes que desenvolveram DRFC do que nos que não desenvolveram a doença em T0 (14,37 ± 1,22 kg/m2 vs. 18,13 ± 3,65 kg/m2; p = 0,049) e em T1 (14,81 ± 0,67 kg/m2 vs. 18,71 ± 3,46 kg/m2; p = 0,022). A análise de subgrupo em relação aos resultados do TOTG em T1 entre anormalidades glicêmicas (DRFC + IGT) e NGT mostrou diferença significativa nos valores de IMC apenas no final do período de acompanhamento. No entanto, considerando a classificação baseada no CGM em T0 (mas não a classificação baseada no TOTG), a análise de subgrupos mostrou valores brutos e escores z de IMC significativamente menores que foram mantidos de T0 a T1. Curiosamente, em relação à classificação do TOTG (IGT vs. NGT) em T0, não foram observadas diferenças significativas no VEF1 ou no IMC (Tabela S2).
Uma análise de regressão logística foi realizada para determinar o efeito do tempo, ajustado para variáveis independentes, no desenvolvimento do DRFC. Os participantes classificados como tendo IGT (no TOTG) tiveram maior chance de desenvolver DRFC (OR = 21,67; IC95%: 7,03-67,36; p < 0,01), enquanto essa chance foi menor entre os participantes com NGT (OR = 1,84; IC95%: 1,06-3,19; p = 0,031). De acordo com a análise logística univariada, sexo masculino, homozigose para p.Phe508del e IMC apresentaram relação significativa com o desenvolvimento de DRFC (Tabela 3). No entanto, a análise de regressão logística multivariada não mostrou associações significativas (dados não apresentados).
DISCUSSÃO Este estudo unicêntrico foi realizado para comparar a capacidade do TOTG com a do CGM em prever o aparecimento de DRFC e comprometimento clínico em pacientes com FC. Na população estudada, os resultados anormais de CGM baseados nos pontos de corte da American Diabetes Association(15) não apresentaram associação com aumento da taxa de DRFC ou declínio do VEF1 ao longo de uma média de 3,1 anos de acompanhamento.
Embora o TOTG seja o padrão ouro recomendado para o diagnóstico de DRFC, não é uma ferramenta ideal, pois os valores de corte são extrapolados do modelo de diabetes tipo 2 do adulto baseado na prevenção de complicações microvasculares, que não são as principais causas de morte em indivíduos com FC. Além disso, diabetes tipo 2 não é o mesmo que DRFC.(23) Há relatos de uma considerável falta de adesão aos TOTG.(5) Portanto, métodos alternativos de rastreamento estão sendo investigados, especialmente aqueles que possam ser relacionados com os desfechos clínicos de pacientes com FC.
Um estudo mostrou que o CGM foi útil para o diagnóstico de DRFC e como indicação para início precoce da insulinoterapia, embora os resultados do TOTG não tenham sido confirmatórios.(24) Nosso estudo não conseguiu mostrar essa relação, embora a disglicemia detectada pelas leituras do CGM tenha sido capaz de identificar comprometimento precoce do IMC em nossos pacientes. Essa diferença pode ter ocorrido em virtude do menor período de acompanhamento em nosso estudo e também do menor tempo de uso do CGM por causa do modelo de dispositivo utilizado em nossa coorte.
Em nosso estudo, quando as anormalidades glicêmicas (DRFC e IGT) foram agrupadas, valores de IMC e escores z de IMC menores no início do estudo e no final do período de acompanhamento foram identificados por meio dos resultados do CGM, mas não dos resultados do TOTG do início do estudo. Um estudo relatou diminuição da função pulmonar e aumento da taxa de infecções por Pseudomonas aeruginosa em pacientes com picos ≥ 200 mg/dL no CGM, embora não houvesse diferença detectável no IMC.(25)
Outro estudo avaliou 25 crianças com FC e constatou que proporção de tempo ≥ 4,5% com níveis de glicose > 140 mg/dL no CGM apresentou associação com declínio da função pulmonar e ganho de peso nos últimos 12 meses.(26) Nosso estudo incluiu uma série de casos mais robusta com um período maior de acompanhamento e detectou menor IMC em pacientes com picos ≥ 140 mg/dL durante o CGM; no entanto, não foram encontradas associações de picos, picos por dia, ASC e proporção de tempo com níveis de glicose ≥ 140 mg/dL com VEF1. A deterioração do VEF1 e do estado nutricional ocorre anos antes do diagnóstico de DRFC.(2) Nesse contexto, identificamos que os pacientes que desenvolveram DRFC apresentaram menor VEF1 em T1, mas não em T0, e também menor IMC tanto em T0 quanto em T1 em nossa amostra. Embora a métrica > 10% do tempo com níveis de glicose ≥ 140 mg/dL no CGM esteja sendo utilizada, ela ainda não foi incorporada às diretrizes e, portanto, não foi considerada para avaliação.
Apesar da ausência de diferenças significativas, todos os pacientes que desenvolveram DRFC eram homozigotos para p.Phe508del e portadores de IP e foram classificados como tendo doença “grave” (≤ 40% do VEF1 previsto) na espirometria e como abaixo do peso (IMC) em T1. Isso está de acordo com a literatura disponível.(1) Além disso, o declínio do VEF1 entre T1 e T0 foi maior naqueles classificados como tendo DRFC de acordo com a classificação do CGM do que naqueles classificados como tendo IGT e NGT. No entanto, esse declínio não foi significativo em nosso estudo, o que pode ser devido ao pequeno tamanho da amostra.
Ser do sexo feminino é considerado fator de risco para DRFC, embora a fisiopatologia a ele relacionada não seja bem compreendida.(1) Todos os pacientes que desenvolveram DRFC eram do sexo masculino; assim, de acordo com a análise de regressão logística univariada, ser do sexo masculino pareceu ser um potencial preditor. No entanto, na análise multivariada, a significância desapareceu após o ajuste.
A hipoglicemia durante o TOTG pode indicar desregulação da secreção de insulina e pode representar um estágio anterior ao aparecimento do DRFC.(27) Os resultados de nosso estudo de CGM mostraram que os eventos hipoglicêmicos não apresentaram relação com risco aumentado de DRFC durante o período médio de acompanhamento de 3,1 anos. Radike et al. relataram achados semelhantes.(7) No entanto, a prevalência de hipoglicemia foi maior (32%) em nosso estudo. Relatou-se que a prevalência de hipoglicemia (< 50 mg/dL) em pacientes com FC durante o TOTG foi de 15%,(6) embora essa porcentagem possa ser atribuída aos menores valores de corte utilizados no estudo citado. Além disso, a idade dos pacientes variou de 8 a 31 anos, enquanto, em nosso estudo, variou de 10,0 a 19,9 anos. Portanto, as discrepâncias nos valores de corte e métodos podem ter contribuído para as maiores taxas de prevalência em nosso estudo.
Apesar da maior sensibilidade do CGM, sua acurácia tem sido questionada em relação à precisão na detecção de hipoglicemia consistente e à falta de diretrizes de consenso, pois não há dados ligando o CGM a desfechos em longo prazo em pacientes com FC.(28) Embora o CGM não tenha conseguido prever o aparecimento de DRFC com base na extrapolação dos critérios utilizados para o TOTG,(15) 4 pacientes com valores glicêmicos ≥ 200 mg/dL foram identificados pelo CGM, mas não pelo TOTG, e nenhum dos participantes que permaneceram com valores de glicose intersticial < 140 mg/dL no CGM evoluiu para DRFC durante o período estudado, deixando a questão em aberto se esses pacientes podem deixar de realizar TOTG. Gojsina et al.(24) mostraram que o CGM pode ter maior sensibilidade, uma vez que pacientes com DRFC diagnosticados por CGM apresentaram níveis de hemoglobina A1c significativamente menores em comparação com aqueles diagnosticados por TOTG.
Dado o seu custo, o CGM pode não estar disponível em todos os serviços para uso rotineiro e poderia ser considerado em indivíduos que não podem ser submetidos ao TOTG e em pacientes sintomáticos com NGT. O CGM é uma ferramenta válida para a detecção de disglicemia na população com FC, e estudos anteriores com maior duração de CGM foram capazes de demonstrar uma associação entre a disglicemia detectada pelo CGM e os desfechos clínicos da FC.(24) O CGM trata da vida diária e não de uma situação controlada como faz o TOTG. O CGM para verificação do metabolismo da glicose pode ser equiparado ao monitoramento ambulatorial da pressão arterial de 24 h para hipertensão.
O CGM pode detectar anormalidades glicêmicas não detectadas pelo TOTG.(23,24) Em nosso estudo, essas anormalidades foram associadas a comprometimento precoce do IMC, embora não tenham sido relacionadas com a definição atual de aparecimento de DRFC com base na classificação do TOTG.(15) Talvez o motivo pelo qual o CGM não conseguiu prever o aparecimento de DRFC seja que os valores de corte do TOTG adotados(15) podem ter sido inadequados e/ou o fato de que o CGM e o TOTG são ferramentas diferentes, tanto do ponto de vista técnico quanto interpretativo. Além disso, de acordo com a Endocrine Society, não há evidências suficientes para o estabelecimento de um valor ideal de glicemia pós-prandial.(24,29)
Apenas 3 de nossos participantes desenvolveram DRFC; assim, não foi possível fazer qualquer afirmação sobre valores de corte, mas recomendamos que futuros estudos multicêntricos avaliem valores de CGM entre 140 e 200 mg/dL para a determinação de valores de corte adequados, uma vez que há estudos mostrando associações entre valores dentro dessa faixa e desfechos clínicos.(30,31) Ainda não está claro se uma única variável de CGM ou uma combinação dessas variáveis poderia prever desfechos clinicamente significativos da FC e potencialmente reformular o conceito de DRFC.
Além disso, sabe-se que pacientes com DRFC precisam de insulina, mas não se sabe se pacientes com FC sem diabetes evidente, mas com anormalidades glicêmicas detectadas por CGM poderiam se beneficiar do uso de insulina.(32) No entanto, Gojsina et al.(24) mostraram que pacientes com DRFC diagnosticados por CGM se beneficiam da insulinoterapia com melhoras no escore z de IMC. Para a prática clínica, a ferramenta ideal seria capaz de prever pior evolução clínica em curto/médio prazo, e, em nossa opinião, o período de acompanhamento adotado cumpre esse objetivo. Então, não devem ser utilizados os mesmos critérios diagnósticos para indivíduos com FC e sem FC, pois a principal causa de mortalidade em pacientes com FC não apresenta relação com complicações microvasculares, mas sim com o agravamento da doença pulmonar. O estabelecimento de uma ferramenta que apresente correlação com o comprometimento clínico na FC, principalmente com função pulmonar e IMC, poderia permitir uma intervenção precoce e gerar economia nos custos da saúde pública, uma vez que a deterioração das condições clínicas leva a maior número de internações, terapias mais agressivas e aumento da necessidade de oxigenoterapia e transplantes pulmonares, além das implicações para a qualidade de vida e a sobrevida dos pacientes com FC.
Os pontos fortes do nosso estudo incluem o grande tamanho da amostra de um único centro de referência em FC, o desenho prospectivo, a coleta de dados padronizada e uma coorte pediátrica puberal com alta miscigenação. No entanto, certas limitações devem ser reconhecidas. Como o DRFC está relacionado à idade, um período mais longo de acompanhamento aumentaria o número de pacientes diagnosticados com essa entidade. O uso arbitrário dos valores de corte do TOTG para classificar os resultados do CGM é outra limitação. O ideal é que o CGM seja realizado durante e no final do período de acompanhamento, mas, infelizmente, não conseguimos fazê-lo. O dispositivo de CGM disponível para o estudo foi o CGMS Gold® (Medtronic Minimed), que permitiu leituras por um curto período. Embora o tamanho da amostra seja grande para um estudo unicêntrico, o pequeno número de pacientes que desenvolveu DRFC durante o período de acompanhamento limitou nossa capacidade de realizar uma análise de regressão multivariada com o atual conjunto de dados.
Em conclusão, o CGM pode identificar anormalidades glicêmicas não detectadas pelo TOTG e pode ser mais sensível para detecção precoce de reduções no IMC. No entanto, com base em nossos dados, não conseguimos identificar preditores precoces do aparecimento de DRFC entre as variáveis estudadas. Indivíduos com níveis de glicose intersticial < 140 mg/dL no CGM podem não precisar se submeter ao TOTG em curto/médio prazo. Além disso, poderíamos ter uma ferramenta alternativa para aqueles pacientes que não são capazes de realizar o TOTG e para aqueles classificados como tendo NGT no TOTG, mas com má evolução clínica. Diferentes critérios diagnósticos podem ser necessários para a população com FC.
AGRADECIMENTOS Os autores gostariam de agradecer a todos os pacientes com FC que participaram deste estudo e aos membros do Hospital Universitário onde está localizado o Centro de Fibrose Cística o apoio no desenvolvimento desta pesquisa.
CONTRIBUIÇÕES DOS AUTORES MZ: conceituação; curadoria de dados; análise formal; investigação; metodologia; administração do projeto; redação, revisão e edição do manuscrito. FALM: análise formal; metodologia; revisão e edição do manuscrito. AMM: análise formal; revisão e edição do manuscrito. ACG: curadoria de dados; revisão e edição do manuscrito. MSEB: curadoria de dados; revisão e edição do manuscrito. JDR: conceituação; metodologia; administração do projeto; redação, revisão e edição do manuscrito. AFR: conceituação; metodologia; administração do projeto; redação, revisão e edição do manuscrito. Todos os autores aprovaram a versão final a ser publicada e concordaram em se responsabilizar por todos os aspectos do estudo, garantindo que as questões relacionadas à acurácia ou integridade de todas as suas partes foram adequadamente investigadas e resolvidas.
CONFLITO DE INTERESSE Não declarado.
REFERÊNCIAS 1. Moran A, Becker D, Casella SJ, Gottlieb PA, Kirkman MS, Marshall BC, et al. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes Care. 2010;33(12):2677-2683. https://doi.org/10.2337/dc10-1279
2. Lanng S, Thorsteinsson B, Nerup J, Koch C. Influence of the development of diabetes mellitus on clinical status in patients with cystic fibrosis. Eur J Pediatr. 1992;151(9):684-687. https://doi.org/10.1007/BF01957574
3. Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162(3 Pt 1):891-895. https://doi.org/10.1164/ajrccm.162.3.9904075
4. Moran A, Pillay K, Becker D, Granados A, Hameed S, Acerini CL. ISPAD Clinical Practice Consensus Guidelines 2018: Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr Diabetes. 2018;19 Suppl 27:64-74. https://doi.org/10.1111/pedi.12732
5. Cystic Fibrosis Foundation [homepage on the Internet]. Bethesda, Maryland: Cystic Fibrosis Foundation; c2019 [cited 2020 Jan 15]. Cystic Fibrosis Foundation Patient Registry 2018 Annual Data Report. Available from: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2018-Patient-Registry-Annual-Data-Report.pdf
6. Battezzati A, Battezzati PM, Costantini D, Seia M, Zazzeron L, Russo MC, et al. Spontaneous hypoglycemia in patients with cystic fibrosis. Eur J Endocrinol. 2007;156(3):369-376. https://doi.org/10.1530/eje.1.02344
7. Radike K, Molz K, Holl RW, Poeter B, Hebestreit H, Ballmann M. Prognostic relevance of hypoglycemia following an oral glucose challenge for cystic fibrosis-related diabetes. Diabetes Care. 2011;34(4):e43. https://doi.org/10.2337/dc10-2286
8. Haliloglu B, Gokdemir Y, Atay Z, Abali S, Guran T, Karakoc F, et al. Hypoglycemia is common in children with cystic fibrosis and seen predominantly in females. Pediatr Diabetes. 2017;18(7):607-613. https://doi.org/10.1111/pedi.12470
9. Dobson L, Sheldon CD, Hattersley AT. Validation of interstitial fluid continuous glucose monitoring in cystic fibrosis. Diabetes Care. 2003;26(6):1940-1941. https://doi.org/10.2337/diacare.26.6.1940-a
10. O’Riordan SM, Hindmarsh P, Hill NR, Matthews DR, George S, Greally P, et al. Validation of continuous glucose monitoring in children and adolescents with cystic fibrosis: a prospective cohort study. Diabetes Care. 2009;32(6):1020-1022. https://doi.org/10.2337/dc08-1925
11. Armaghanian N, Brand-Miller JC, Markovic TP, Steinbeck KS. Hypoglycaemia in cystic fibrosis in the absence of diabetes: A systematic review. J Cyst Fibros. 2016;15(3):274-284. https://doi.org/10.1016/j.jcf.2016.02.012
12. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017;40(12):1631-1640. https://doi.org/10.2337/dc17-1600
13. Bismuth E, Laborde K, Taupin P, Velho G, Ribault V, Jennane F, et al. Glucose tolerance and insulin secretion, morbidity, and death in patients with cystic fibrosis. J Pediatr. 2008;152(4):540-545.e1. https://doi.org/10.1016/j.jpeds.2007.09.025
14. GIBSON LE, COOKE RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959;23(3):545-549. https://doi.org/10.1542/peds.23.3.545
15. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5-S20. https://doi.org/10.2337/diacare.26.2007.s5
16. World Health Organization. International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation [monograph on the Internet]. Geneva: World Health Organization; 2006 [Cited 2018 Nov 12]. Available from: https://apps.who.int/iris/handle/10665/43588
17. Miller M, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. https://doi.org/10.1183/09031936.05.00034805
18. Rodrigues JC, Cardieri JM, Bussamra MH, Nakaie CM, Almeida MB, Silva FL, et al. Provas de função pulmonar em crianças e adolescentes. J Pneumol. 2002;28(Suppl 3):207-221.
19. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76-85. https://doi.org/10.1111/j.1651-2227.2006.tb02378.x
20. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291-303. https://doi.org/10.1136/adc.44.235.291
21. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13-23. https://doi.org/10.1136/adc.45.239.13
22. Borowitz D, Baker SS, Duffy L, Baker RD, Fitzpatrick L, Gyamfi J, et al. Use of fecal elastase-1 to classify pancreatic status in patients with cystic fibrosis. J Pediatr. 2004;145(3):322-326. https://doi.org/10.1016/j.jpeds.2004.04.049
23. Granados A, Chan CL, Ode KL, Moheet A, Moran A, Holl R. Cystic fibrosis related diabetes: Pathophysiology, screening and diagnosis. J Cyst Fibros. 2019;18 Suppl 2:S3-S9. https://doi.org/10.1016/j.jcf.2019.08.016
24. Gojsina B, Minic P, Todorovic S, Soldatovic I, Sovtic A. Continuous Glucose Monitoring as a Valuable Tool in the Early Detection of Diabetes Related to Cystic Fibrosis. Front Pediatr. 2021;9:659728. https://doi.org/10.3389/fped.2021.659728
25. Leclercq A, Gauthier B, Rosner V, Weiss L, Moreau F, Constantinescu AA, et al. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J Cyst Fibros. 2014;13(4):478-484. https://doi.org/10.1016/j.jcf.2013.11.005
26. Hameed S, Morton JR, Jaffé A, Field PI, Belessis Y, Yoong T, et al. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care. 2010;33(2):221-226. https://doi.org/10.2337/dc09-1492
27. Tofé S, Moreno JC, Máiz L, Alonso M, Escobar H, Barrio R. Insulin-secretion abnormalities and clinical deterioration related to impaired glucose tolerance in cystic fibrosis. Eur J Endocrinol. 2005;152(2):241-247. https://doi.org/10.1530/eje.1.01836
28. Ode KL, Moran A. New insights into cystic fibrosis-related diabetes in children. Lancet Diabetes Endocrinol. 2013;1(1):52-58. https://doi.org/10.1016/S2213-8587(13)70015-9
29. Leahy JJL, Aleppo G, Fonseca VA, Garg SK, Hirsch IB, McCall AL, et al. Optimizing Postprandial Glucose Management in Adults With Insulin-Requiring Diabetes: Report and Recommendations. J Endocr Soc. 2019;3(10):1942-1957. https://doi.org/10.1210/js.2019-00222
30. Taylor-Cousar JL, Janssen JS, Wilson A, Clair CG, Pickard KM, Jones MC, et al. Glucose >200 mg/dL during Continuous Glucose Monitoring Identifies Adult Patients at Risk for Development of Cystic Fibrosis Related Diabetes. J Diabetes Res. 2016;2016:1527932. https://doi.org/10.1155/2016/1527932
31. Chan CL, Vigers T, Pyle L, Zeitler PS, Sagel SD, Nadeau KJ. Continuous glucose monitoring abnormalities in cystic fibrosis youth correlate with pulmonary function decline. J Cyst Fibros. 2018 Nov;17(6):783-790. https://doi.org/10.1016/j.jcf.2018.03.008
32. Pu MZ, Christensen-Adad FC, Gonçalves AC, Minicucci WJ, Ribeiro JD, Ribeiro AF. Insulin therapy in patients with cystic fibrosis in the pre-diabetes stage: a systematic review. Rev Paul Pediatr. 2016;34(3):367-373. https://doi.org/10.1016/j.rppede.2016.03.006








