ABSTRACT
Objective: To evaluate small airway disease in COVID-19 patients using the prevalence of air trapping (AT) and correlating it with clinical outcomes. The relationship between CT-based opacities in small blood vessels and ventilation in patients with SARS-CoV-2 pneumonia was also assessed. Methods: We retrospectively included 53 patients with positive RT-PCR results for SARS-CoV-2 between March and April of 2020. All subjects underwent HRCT scanning, including inspiratory and expiratory acquisitions. Subjects were divided into two groups based on visual identification of AT. Small blood vessel volumes were estimated by means of cross-sectional areas < 5 mm2 (BV5) derived from automated segmentation algorithms. Mixed-effect models were obtained to represent the BV5 as a function of CT-based lobar opacities and lobar ventilation. Results: Of the 53 participants, AT was identified in 23 (43.4%). The presence of AT was associated with increased SpO2 at admission (OR = 1.25; 95% CI, 1.07-1.45; p = 0.004) and reduced D-dimer levels (OR = 0.99; 95% CI, 0.99-0.99; p = 0.039). Patients with AT were less likely to be hospitalized (OR = 0.27; 95% CI, 0.08-0.89; p = 0.032). There was a significant but weak inverse correlation between BV5 and CT-based lobar opacities (R2 = 0.19; p = 0.03), as well as a nonsignificant and weak direct correlation between BV5 and lobar ventilation (R2 = 0.08; p = 0.54). Conclusions: AT is a common finding in patients with COVID-19 that undergo expiratory CT scanning. The presence of AT may correlate with higher SpO2 at admission, lower D-dimer levels, and fewer hospitalizations when compared with absence of AT. Also, the volume of small pulmonary vessels may negatively correlate with CT opacities but not with lobar ventilation.
Keywords:
SARS-CoV-2; COVID-19; Tomography, X-ray.
RESUMO
Objetivo: Avaliar a doença das pequenas vias aéreas em pacientes com COVID-19 por meio da prevalência de aprisionamento aéreo (AA) e sua correlação com desfechos clínicos. Também foi avaliada a relação entre opacidades tomográficas nos pequenos vasos sanguíneos e ventilação em pacientes com pneumonia por SARS-CoV-2. Métodos: Foram incluídos, retrospectivamente, 53 pacientes com teste de RT-PCR positivo para SARS-CoV-2 entre março e abril de 2020. Todos os indivíduos foram submetidos à TCAR, incluindo aquisições inspiratórias e expiratórias. Os indivíduos foram divididos em dois grupos com base na identificação visual de AA. Os volumes dos pequenos vasos sanguíneos foram estimados por meio de seções transversais < 5 mm2 (VS5) derivadas de algoritmos automatizados de segmentação. Modelos de efeito misto foram obtidos para representar o VS5 em função das opacidades lobares tomográficas e da ventilação lobar. Resultados: Identificou-se AA em 23 (43,4%) dos 53 participantes. A presença de AA apresentou associação com SpO2 elevada na admissão (OR = 1,25; IC95%: 1,07-1,45; p = 0,004) e níveis reduzidos de dímero D (OR = 0,99; IC95%: 0,99-0,99; p = 0,039). Pacientes com AA apresentaram menor probabilidade de hospitalização (OR = 0,27; IC95%: 0,08-0,89; p = 0,032). Houve correlação inversa significativa, mas fraca, entre VS5 e opacidades lobares tomográficas (R2 = 0,19; p = 0,03) e correlação direta não significativa e fraca entre VS5 e ventilação lobar (R2 = 0,08; p = 0,54). Conclusões: AA é um achado comum em pacientes com COVID-19 submetidos à TC expiratória. A presença de AA pode apresentar correlação com SpO2 elevada na admissão, níveis reduzidos de dímero D e menor probabilidade de hospitalização. Além disso, o volume dos pequenos vasos pulmonares pode apresentar correlação negativa com opacidades tomográficas, mas não com ventilação lobar.
Palavras-chave:
SARS-CoV-2; COVID-19; Tomografia computadorizada por raios X.
INTRODUCTION The SARS-CoV-2 pneumonia course is characterized by severe hypoxemia with preserved lung compliance. (1,2) The underlying causes of COVID-19-related acute respiratory failure are vascular injury and vasoconstriction, with microvascular injury causing the pulmonary exudate leak, which is characteristic of SARS-CoV-2 pneumonia.(1,2) The presence of inflammatory exudates in the airways leading to airway remodeling and destruction of alveolar walls has been described as the pathophysiology of other pulmonary diseases such as COPD and asthma. This damage to the small airways leads to airflow obstruction and air trapping (AT), which are markers of COVID-19 severity and prognosis.(3,4) AT has also been reported in pulmonary hypertension due to an increase in the caliber of arteries in areas of increased attenuation (hyperemia) when compared with smaller vessels in areas of low attenuation (oligemia).(5) A group of authors reported that the presence of AT was significantly more common in patients with COVID-19 admitted to the ICU or who died. (6) Thus, it is reasonable to question whether COVID-19 could result in small airway damage and AT and whether microvascular thrombosis may contribute to bronchiolar constriction or small airway disease.(7)
Chest CT has played a significant role in the COVID-19 evaluation, and abnormal CT findings have been reported in up to 90% of hospitalized patients.(8,9) The predominance of ground-glass opacities (GGO) is one of the most common patterns of SARS-CoV-2 pneumonia. (8,9) Although imaging cannot diagnose SARS-CoV-2, it can assess the severity and extension of lower respiratory tract involvement, as well as provide alternative diagnoses and concomitant pathologies, such as pulmonary embolism. (10,11) However, the role of expiratory CT acquisitions in COVID-19 remains unclear, and most imaging centers include only inspiratory phases to avoid patient exposure to additional radiation doses.(12) Thus, the prevalence of AT in COVID-19 patients might be underestimated. Some follow-up studies of SARS-CoV-2 pneumonia have demonstrated that AT may also be identified months after the infection.(13)
Quantitative CT imaging has been used to predict clinical outcomes of several pulmonary diseases, and the percentage of AT has been used as one of the quantitative CT tools in the evaluation of asthma, COPD, and interstitial lung diseases.(14-17) Regarding COVID-19, few articles have evaluated quantitative CT findings as markers of disease progression and prognosis. Increasing percentages of consolidation and GGO on chest CT have been found to estimate the risk of clinical deterioration or death in patients with COVID-19 pneumonia.(18) Also, a quantitative decrease in well-aerated lung volume has been reported to predict adverse outcomes in COVID-19.(19) Thus, the purpose of the present study was to evaluate small airway disease in COVID-19 patients by using the prevalence of AT and correlating it with clinical outcomes. The relationship between small blood vessels with CT opacities and ventilation in SARS-CoV-2 pneumonia patients was also assessed.
METHODS The local institutional review board approved this cross-sectional retrospective study, and written consent was waived. We retrospectively included patients with positive SARS-CoV-2 RT-PCR results from throat swabs or lower respiratory tract samples between March 21, 2020 and April 20, 2020, in three hospitals. Patients should have undergone chest CT in the same period of a positive RT-PCR test.
Volumetric HRCT scans were obtained from all subjects, including acquisitions at full inspiration and the end of a normal expiration. The expiratory CT acquisition phase was already part of our institutional CT protocol to assess pulmonary infections before the COVID-19 pandemic. All CT scans were performed with a peak tube voltage of 120 kVp and a fixed tube current of 200 mAs for inspiratory CT and of 50 mAs for expiratory CT at a gantry rotation time of 0.5 s. The reconstructed slice thickness was 1.25 mm using a 64-slice CT scanner (LightSpeed VCT; GE Healthcare, Chicago, IL, USA).
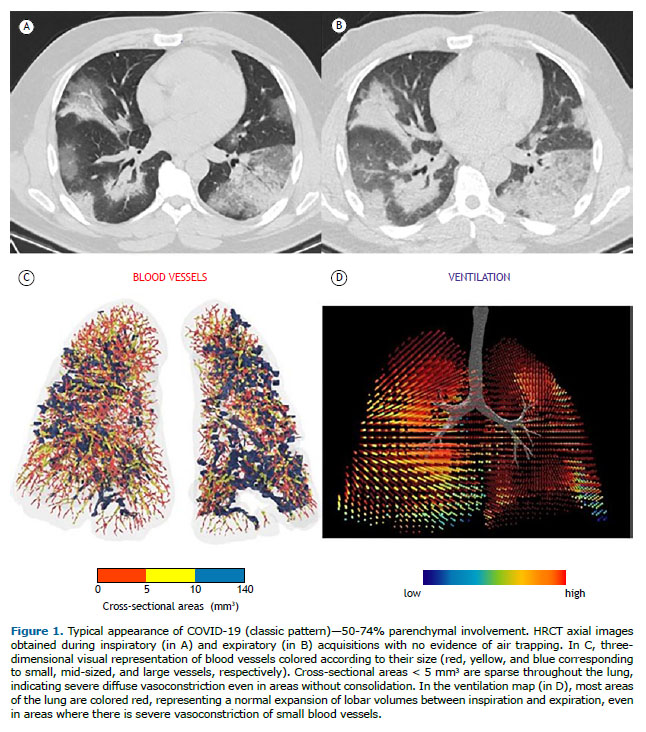
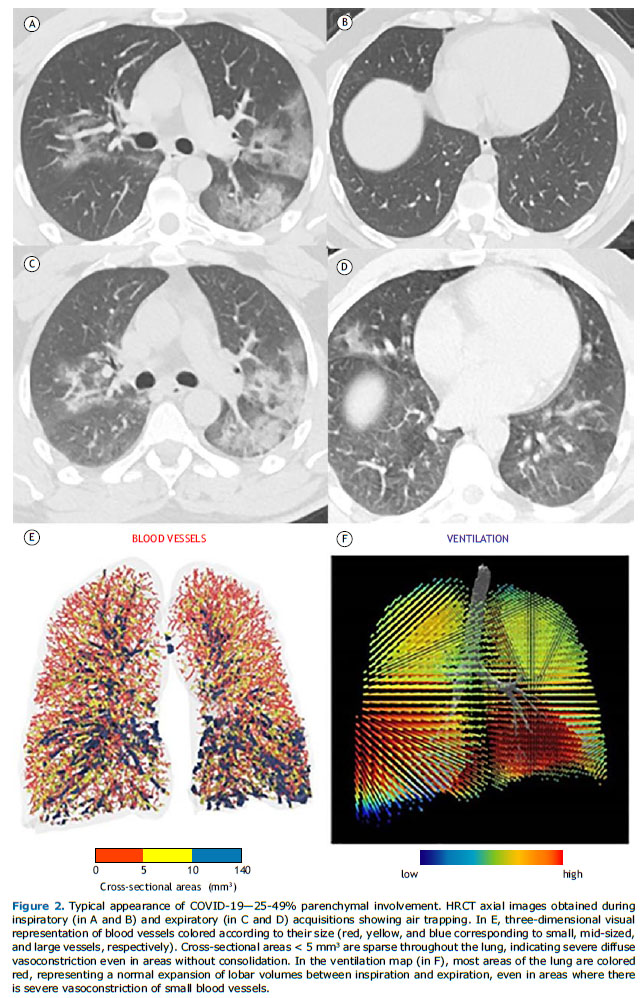
Inspiratory and expiratory HRCT imaging datasets were analyzed using a digital database system (CARESTREAM Vue PACS, version 12.2.1.2; Carestream Health, Rochester, NY, USA), and the two radiologists (with 5 and 9 years of experience) who performed the analysis were blinded to clinical and laboratory results of the patients. The use of intravenous contrast media was requested at the discretion of the attending physician or radiologist. HRCT findings were described using the international standard nomenclature defined by the Fleischner Society glossary(20) and the British Society of Thoracic imaging classification of COVID-19 pneumonia (classic, probable, indeterminate, or non-COVID-19).(21) A semiquantitative score was used in order to estimate the parenchymal involvement with GGO and consolidation at inspiratory acquisitions by using the following lesion extension ranges: 0-24%; 25-49%; 50-74%; and 75-100%. Patients were divided into two groups based on the presence of AT, which was defined as parenchymal areas with less than the normal increase in attenuation and lack of volume reduction on end-expiration CT scans.(20) Regional AT was considered present when at least three secondary lobules were involved, as previously described.(22)
Data on airways and small vessels were also post-processed using functional respiratory imaging analysis, a technique to assess airway morphology that has been extensively validated in humans.(23-25) Three-dimensional reconstructions of the lung and pulmonary vasculature were created using a software program (FLUIDDA, Kontich, Belgium) approved by the US Food and Drug Administration. Using an automated blood vessel segmentation algorithm previously described,(26,27) we calculated the volume of blood contained in vessels in three ranges of cross-sectional areas: < 5 mm3 (BV5); 5-10 mm3 (BV5-BV10), and > 10 mm3 (BV10). Three-dimensional visual representations of the blood vessels were created, and they were colored according to their size (Figures 1 and 2). From the data derived from gated inspiratory and expiratory CT scans, ventilation maps were created by assuming that regional changes in lung volume would relate to regional ventilation, as previously described.(28) Mixed-effect models were also obtained to represent the predicted percentage of BV5 as a function of volume of CT-based opacities within a lobe and lobar ventilation.
Demographic, clinical, and laboratory variables were collected from the electronic medical records of the institutions. Such parameters were based on previous investigations that found correlations between such variables and the severity of respiratory failure in patients with COVID-19.(29,30)
Data were presented as absolute and relative frequencies, as well as means and standard deviations or medians and interquartile ranges. The Shapiro-Wilk test was used to assess the normality of data distribution. We evaluated associations between variables with chi-squared tests. For comparing continuous variables, the Mann-Whitney test was used. The Student’s t-test was used for continuous variables for two-group comparisons. All tests were two-tailed with a level of significance set at p < 0.05. Statistical analyses were performed using Stata statistical software package, version 15 (StataCorp LP, College Station, TX, USA).
RESULTS In total, 53 patients were included, and AT was identified in 23 patients (43.4%). There were no significant differences between the patients with AT (AT group) and those without AT (non-AT group) regarding their baseline characteristics (Table 1). Both groups presented similar prevalences of comorbidities that cause AT, such as asthma and COPD. In accordance with the imaging classification of COVID-19 pneumonia,(21) the non-COVID-19 pattern was identified in 6 and 6 patients in the AT and non-AT groups, respectively (Table 1). Although most patients in both groups presented with the classic/probable COVID-19 pattern on CT (Figure 1), no significant differences were found in the prevalence of classic/probable or indeterminate COVID-19 patterns on CT between the groups (p = 0.196). Most patients included in our study presented with < 50% of lung involvement on their CT scans. Although the prevalences of lung involvement ≥ 50% were different between the groups, they were not statistically significant (p = 0.622).
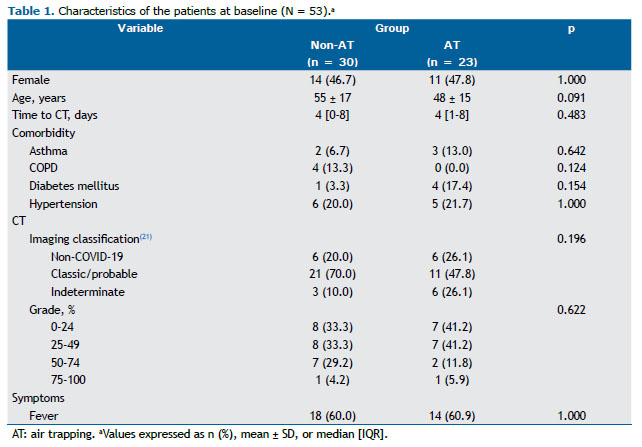
Table 2 summarizes the comparison of outcomes between the groups. There were significant differences between the groups in SpO2 at admission and D-dimer levels, as well as the need for hospitalization. In the initial evaluation, the non-AT group presented lower SpO2 (p = 0.012) and higher D-dimer levels (p = 0.001) that did the AT group. A greater proportion of patients in the non-AT group required hospitalization when compared with those in the AT group (73.3% vs. 43.5%; p = 0.028).
The logistic regression univariate analysis compared the outcomes between the groups. The presence of AT was associated with a 25% increase in SpO2 at admission (OR = 1.25; 95% CI, 1.07-1.45; p = 0.004) and lower D-dimer levels (OR = 0.99; 95% CI, 0.99-0.99; p = 0.039). Also, patients with AT were less likely to be hospitalized (OR = 0.27; 95% CI, 0.08-0.89; p = 0.032).
There was a significant but weak inverse correlation between BV5 and CT-based opacities (R2 = 0.19; p = 0.03; Figure 3). Also, there was a nonsignificant and weak direct correlation between BV5 and lobar ventilation (R2 = 0.08; p = 0.54; Figure 4).
DISCUSSION In the present study, we found that AT was a common finding among patients that underwent CT scanning with additional expiratory acquisitions. There was a significant difference between patients with and without AT in SpO2 at admission, D-dimer levels, and hospitalization that was confirmed in the univariate regression analysis.
Previous studies have reported prevalences of AT up to 64% among subjects with normal pulmonary function.(31) Such a prevalence is comparable to the one found in our study (43.4%). Likewise, only 17% of the included patients had comorbidities that could lead to AT, such as asthma and COPD.
Loss of peripheral pulmonary vessels has been reported to correlate with worse clinical outcomes in asthma, COPD, and pulmonary hypertension.(32-35) On CT, peripheral vascular pruning can be represented as lower volumes of BV5. Our study found that the proportion of BV5 within a lung lobe was inversely correlated with the volume of CT-based lobar opacities but not with lobar ventilation. Hence, severely constricted areas were still well ventilated. This finding corroborates the hypothesis of hypoxia with preserved lung compliance. It also supports Lins et al.,(26) who suggested that COVID-19 is an infectious mimic of idiopathic pulmonary hypertension, since both diseases may present microvascular coagulopathy and increased muscularization of pulmonary arteries.
Serum D-dimer is a marker of microvascular injury. In patients with COVID-19, higher D-dimer levels are related to worse outcomes, respiratory failure, and pulmonary embolism.(29,30,36) This could be related to higher activation of blood coagulation secondary to a systemic inflammatory response syndrome or a direct consequence of the SARS-CoV-2 infection. Our data suggest that patients with AT have lower vascular damage and better outcomes. This could be related to the fact that patients with AT on chest CT have “more” airway disease than small vessel disease. Thus, we hypothesized that there might be two phenotypes of SARS-CoV-2 pneumonia. First, the small-vessel phenotype, characterized by severe vasoconstriction and microvascular coagulopathy, which results in hypoxemia with preserved lung compliance. Second, the small-airway phenotype, characterized by small airway damage, represented on imaging by the presence of AT. These findings have to be further confirmed by larger studies, and it is still soon to make any recommendations on whether expiratory acquisitions should be included in CT scanning of all patients with COVID-19 pneumonia.
In a previous study of patients clinically diagnosed with COVID-19,(6) AT was more prevalent in those who had been admitted to the ICU or died. However, that study had several limitations that should be acknowledged, which might explain the difference between their results and ours. First, 34.4% of their sample had a negative SARS-CoV-2 RT-PCR test, which weakens the association between AT and worse outcomes because non-COVID-19 cases could have been included. In our study, all patients should have a positive SARS-CoV-2 RT-PCR result. Second, they included a small sample size of patients with poor outcomes.
Our study has some limitations. First, we acknowledge the preliminary nature of our findings, including the lack of a severe COVID-19 comparison group. Secondly, we could only include a small sample size, mainly due to concerns about the patients’ exposure to additional radiation with no clear clinical benefits. These preliminary results might encourage further investigations with larger sample sizes to be carried out in the future.
In summary, we found that AT is a common finding in patients with COVID-19 who undergo expiratory CT acquisitions. The presence of AT may correlate with increased SpO2 at admission, reduced serum D-dimer levels, and a decreased likelihood of hospitalization. Also, the volume of small pulmonary vessels may negatively correlate with CT opacities but not with lobar ventilation, suggesting that severely constricted areas are still well ventilated in COVID-19 patients.
AUTHOR CONTRIBUTIONS BH: guarantor. RDC and BH: study design. RDC, NV, GW, TM, SPLA, MZ, JDB, BRL, EM, and BH: manuscript drafting. RDC, JDB, and BRL: data acquisition. RDC, NV, TM, SPLA, MZ, EM, BH GW, and BH: data interpretation. RDC, NV, GW, TM, SPLA, MZ, JDB, BRL, EM, and BH: critical review and approval of the final manuscript.
CONFLICT OF INTEREST None declared.
REFERENCES 1. Higham A, Mathioudakis A, Vestbo J, Singh D. COVID-19 and COPD: a narrative review of the basic science and clinical outcomes. Eur Respir Rev. 2020;29(158):200199. https://doi.org/10.1183/16000617.0199-2020
2. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med. 2020 Apr;8(4):e26]. Lancet Respir Med. 2020;8(5):475-481. https://doi.org/10.1016/S2213-2600(20)30079-5
3. MacNee W. Pathology, pathogenesis, and pathophysiology. BMJ. 2006;332(7551):1202-4. https://doi.org/10.1136/bmj.332.7551.1202
4. Perez T, Chanez P, Dusser D, Devillier P. Small airway impairment in moderate to severe asthmatics without significant proximal airway obstruction. Respir Med. 2013;107(11):1667-1674. https://doi.org/10.1016/j.rmed.2013.08.009
5. Ussavarungsi K, Lee AS, Burger CD. Mosaic Pattern of Lung Attenuation on Chest CT in Patients with Pulmonary Hypertension. Diseases. 2015;3(3):205-212. https://doi.org/10.3390/diseases3030205
6. Erturk SM, Durak G, Ayyildiz H, Comert RG, Medetalibeyoglu A, Senkal N, et al. Covid-19: Correlation of Early Chest Computed Tomography Findings With the Course of Disease. J Comput Assist Tomogr. 2020;44(5):633-639. https://doi.org/10.1097/RCT.0000000000001073
7. Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, et al. Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations. Am J Respir Crit Care Med. 2020;202(5):690-699. https://doi.org/10.1164/rccm.202004-1412OC
8. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/NEJMoa2002032
9. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296(2):E32-E40. https://doi.org/10.1148/radiol.2020200642
10. Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, et al. The Role of Chest Imaging in Patient Management During the COVID-19 Pandemic: A Multinational Consensus Statement From the Fleischner Society. Chest. 2020;158(1):106-116. https://doi.org/10.1016/j.chest.2020.04.003
11. Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020;295(1):202-207. https://doi.org/10.1148/radiol.2020200230
12. Kwee TC, Kwee RM. Chest CT in COVID-19: What the Radiologist Needs to Know [published correction appears in Radiographics. 2022 Jan-Feb;42(1):E32]. Radiographics. 2020;40(7):1848-1865. https://doi.org/10.1148/rg.2020200159
13. Ebner L, Funke-Chambour M, von Garnier C, Ferretti G, Ghaye B, Beigelman-Aubry C. Imaging in the aftermath of COVID-19: what to expect. Eur Radiol. 2021;31(6):4390-4392. https://doi.org/10.1007/s00330-020-07465-6
14. Matsuoka S, Kurihara Y, Yagihashi K, Hoshino M, Watanabe N, Nakajima Y. Quantitative assessment of air trapping in chronic obstructive pulmonary disease using inspiratory and expiratory volumetric MDCT. AJR Am J Roentgenol. 2008;190(3):762-769. https://doi.org/10.2214/AJR.07.2820
15. Hochhegger B, Sanches FD, Altmayer SPL, Pacini GS, Zanon M, Guedes ÁDCB, et al. Air trapping in usual interstitial pneumonia pattern at CT: prevalence and prognosis. Sci Rep. 2018;8(1):17267. https://doi.org/10.1038/s41598-018-35387-3
16. Lee YK, Oh YM, Lee JH, Kim EK, Lee JH, Kim N, et al. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography [published correction appears in Lung. 2008 Jul-Aug;186(4):277] [published correction appears in Lung. 2008 Aug;186(4):277]. Lung. 2008;186(3):157-165. https://doi.org/10.1007/s00408-008-9071-0
17. Hochhegger B, Zanon M, Altmayer S, Pacini GS, Balbinot F, Francisco MZ, et al. Advances in Imaging and Automated Quantification of Malignant Pulmonary Diseases: A State-of-the-Art Review. Lung. 2018;196(6):633-642. https://doi.org/10.1007/s00408-018-0156-0
18. Grodecki K, Lin A, Cadet S, McElhinney PA, Razipour A, Chan C, et al. Quantitative Burden of COVID-19 Pneumonia on Chest CT Predicts Adverse Outcomes: A Post-Hoc Analysis of a Prospective International Registry. Radiol Cardiothorac Imaging. 2020;2(5):e200389. https://doi.org/10.1148/ryct.2020200389
19. Colombi D, Bodini FC, Petrini M, Maffi G, Morelli N, Milanese G, et al. Well-aerated Lung on Admitting Chest CT to Predict Adverse Outcome in COVID-19 Pneumonia. Radiology. 2020;296(2):E86-E96. https://doi.org/10.1148/radiol.2020201433
20. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722. https://doi.org/10.1148/radiol.2462070712
21. Nair A, Rodrigues J, Hare S, Edey A, Devaraj A, Jacob J, et al. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID-19 pandemic. Clin Radiol. 2020;75(5):329-334. https://doi.org/10.1016/j.crad.2020.03.008
22. Karimi R, Tornling G, Forsslund H., Mikko M, Wheelock ÅM, Nyrén S, et al. Differences in regional air trapping in current smokers with normal spirometry. Eur Respir J. 2017;49(1):1600345. https://doi.org/10.1183/13993003.00345-2016
23. De Backer JW, Vos WG, Vinchurkar SC, Claes R, Drollmann A, Wulfrank D, et al. Validation of computational fluid dynamics in CT-based airway models with SPECT/CT. Radiology. 2010;257(3):854-862. https://doi.org/10.1148/radiol.10100322
24. De Backer J, Vos W, Vinchurkar S, Van Holsbeke C, Poli G, Claes R, et al. The effects of extrafine beclometasone/formoterol (BDP/F) on lung function, dyspnea, hyperinflation, and airway geometry in COPD patients: novel insight using functional respiratory imaging. J Aerosol Med Pulm Drug Deliv. 2015;28(2):88-99. https://doi.org/10.1089/jamp.2013.1064
25. Hajian B, De Backer J, Vos W, Van Holsbeke C, Clukers J, De Backer W. Functional respiratory imaging (FRI) for optimizing therapy development and patient care. Expert Rev Respir Med. 2016;10(2):193-206. https://doi.org/10.1586/17476348.2016.1136216
26. Lins M, Vandevenne J, Thillai M, Lavon BR, Lanclus M, Bonte S, et al. Assessment of Small Pulmonary Blood Vessels in COVID-19 Patients Using HRCT. Acad Radiol. 2020;27(10):1449-1455. https://doi.org/10.1016/j.acra.2020.07.019
27. Rahaghi FN, Argemí G, Nardelli P, Domínguez-Fandos D, Arguis P, Peinado VI, et al. Pulmonary vascular density: comparison of findings on computed tomography imaging with histology. Eur Respir J. 2019;54(2):1900370. https://doi.org/10.1183/13993003.00370-2019
28. Bonte S, Lanclus M, Van Holsbeke C, De Backer J. Functional Respiratory Imaging (FRI) shows significant regional ventilation defects in COPD patients as compared to healthy data. Eur Respir J. 2020;56(Suppl. 64):2085. https://doi.org/10.1183/13993003.congress-2020.2085
29. Léonard-Lorant I, Delabranche X, Séverac F, Helms J, Pauzet C, Collange O, et al. Acute Pulmonary Embolism in Patients with COVID-19 at CT Angiography and Relationship to d-Dimer Levels. Radiology. 2020;296(3):E189-E191. https://doi.org/10.1148/radiol.2020201561
30. Zhang MQ, Wang XH, Chen YL, Zhao KL, Cai YQ, An CL, et al. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing [Article in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):215-218.
31. Tanaka N, Matsumoto T, Miura G, Emoto T, Matsunaga N, Ueda K, et al. Air trapping at CT: high prevalence in asymptomatic subjects with normal pulmonary function. Radiology. 2003;227(3):776-785. https://doi.org/10.1148/radiol.2273020352
32. Ash SY, Rahaghi FN, Come CE, Ross JC, Colon AG, Cardet-Guisasola JC, et al. Pruning of the Pulmonary Vasculature in Asthma. The Severe Asthma Research Program (SARP) Cohort. Am J Respir Crit Care Med. 2018;198(1):39-50. https://doi.org/10.1164/rccm.201712-2426OC
33. Estépar RS, Kinney GL, Black-Shinn JL, Bowler RP, Kindlmann GL, Ross JC, et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188(2):231-239. https://doi.org/10.1164/rccm.201301-0162OC
34. Matsuoka S, Washko GR, Dransfield MT, Yamashiro T, San Jose Estepar R, Diaz A, et al. Quantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: correlations with emphysema and airflow limitation. Acad Radiol. 2010;17(1):93-99. https://doi.org/10.1016/j.acra.2009.07.022
35. Matsuoka S, Washko GR, Yamashiro T, Estepar RS, Diaz A, Silverman EK, et al. Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med. 2010;181(3):218-225. https://doi.org/10.1164/rccm.200908-1189OC
Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography. Radiology. 2020;296(3):E186-E188. https://doi.org/10.1148/radiol.2020201544







