ABSTRACT
Objective: To evaluate the evolution of clinical and epidemiological data, as well as data related to diagnosis, staging, treatment, and survival, among patients undergoing curative surgery for lung cancer at a tertiary referral center in the city of São Paulo, Brazil. Methods: This was a retrospective study of cases in the International Association for the Study of Lung Cancer database. We selected only cases of patients undergoing curative surgery between January of 2011 and April of 2018. We determined overall and disease-free survival at 36 months and compared the data between two periods (2011-2014 and 2015-2018). Results: Comparing the two periods (N = 437 cases), we observed trends toward increases in the number of female patients, as well as in the proportions of former smokers (44.09% vs. 53.59%), of patients diagnosed with adenocarcinoma (52.21% vs. 59.72%), and of patients diagnosed at an earlier pathological stage, together with a decrease in 30-day mortality (4.05% vs. 2.39%). There were significant increases in the proportions of cases diagnosed at an earlier clinical stage (p = 0.002) or incidentally (p = 0.003). Although lobectomy was the main surgical technique employed, there was a proportional increase in segmentectomies (2.67% vs. 7.11%; p = 0.026). Overall and disease-free survival rates were 79.4% (95% CI: 74.0-83.9%) and 75.1% (95% CI: 69.1-80.1%), respectively. The difference in overall survival between the periods lost statistical significance when adjusted for pathological stage, the only factor that affected survival (log-rank: p = 0.038 to p = 0.079). Conclusions: The clinical and epidemiological evolution presented in this study corroborates global trends. The decrease in 30-day mortality was probably due to better patient selection and improved surgical techniques.
Keywords:
Lung neoplasms/surgery; Lung neoplasms/epidemiology; Lung neoplasms/therapy; Survival analysis; Thoracic surgery; Thoracic surgery, video-assisted.
RESUMO
Objetivo: Avaliar a evolução de dados clínicos e epidemiológicos, assim como dados sobre diagnóstico, estadiamento, tratamento e sobrevida em pacientes submetidos a tratamento cirúrgico curativo de câncer de pulmão em uma instituição terciária na cidade de São Paulo (SP). Métodos: Estudo retrospectivo baseado nos casos inseridos no banco de dados da International Association for the Study of Lung Cancer submetidos à cirurgia curativa entre janeiro de 2011 e abril de 2018. Determinamos a sobrevida global e livre de doença em 36 meses e comparamos os dados em dois períodos (2011-2014 e 2015-2018). Resultados: Comparando-se os dois períodos (N = 437 casos), houve uma tendência de aumento no número de pacientes do sexo feminino, ex-tabagistas (44,09% vs. 53,59%), com diagnóstico de adenocarcinoma (52,21% vs. 59,72%) e em estádio patológico mais precoce, assim como queda da mortalidade em 30 dias (4,05% vs. 2,39%). Houve aumento significativo de casos em estádio clínico mais precoce (p = 0,002) e diagnosticados incidentalmente (p = 0,003). A lobectomia foi a principal técnica cirúrgica; entretanto, houve aumento de segmentectomias (2,67% vs. 7,11%; p = 0,026). As sobrevidas global e livre de doença foram de 79,4% (IC95%: 74,0-83,9%) e 75,1% (IC95%: 69,1-80,1%), respectivamente. Houve perda de significância estatística na sobrevida global entre os períodos quando ajustada por estadiamento patológico, o único fator a impactar a sobrevida (log-rank: p = 0,038 para p = 0,079). Conclusões: A evolução clínica e epidemiológica apresentada neste estudo corrobora tendências mundiais. A diminuição da mortalidade em 30 dias provavelmente ocorreu devido a melhor seleção de pacientes e melhora da técnica cirúrgica.
Palavras-chave:
Neoplasias pulmonares/cirurgia; Neoplasias pulmonares/epidemiologia; Neoplasias pulmonares/terapia; Análise de Sobrevida; Cirurgia torácica; Cirurgia torácica videoassistida.
INTRODUCTIONLung cancer has evolved from being considered a rare neoplasm in the early 20th century(1) to being one of the leading malignancies in the world today, with an estimated more than 2 million new cases (11.6% of all cancer cases) in 2018. The high lethality of lung cancer underscores the importance of the disease, given that there were an estimated 1.8 million lung cancer deaths in that same year.(2) In Brazil, according to the Brazilian National Cancer Institute, there were an estimated 31,270 new cases of lung cancer in 2018 (18,740 in men and 12,530 in women). In addition, lung cancer is the second leading cancer in men and the fourth leading cancer in women,(3) a similar scenario being seen in the city of São Paulo. (4) In a review article on lung cancer in Brazil, Araújo et al.(5) reported that the age-standardized 5-year survival rate in the country is 18%, which is in line with those reported worldwide, which range from 10% to 20%.(6)
Some aspects of the Brazilian reality make it difficult to apply findings from studies on lung cancer conducted in the United States and Europe. The inequalities between public and private medicine in Brazil, in terms of access to tests and treatment,(5) as well as the high incidence of granulomatous diseases,(7) influence our results. In the scientific literature of Brazil, there are estimates of the incidence of and mortality from lung cancer made by the Brazilian National Cancer Institute. However, other clinical data, as well as observed mortality and survival data, are scarce. Many of the studies conducted in Brazil were published long ago or were single-center studies with no focus on surgically treated lung cancer patients, proving data only related to histology, staging, type of treatment, and survival.(8-13)
The objective of the present study was to evaluate the evolution (from 2011 to 2018) of clinical and epidemiological data on lung cancer patients undergoing surgery with curative intent at a tertiary referral center in the state of São Paulo, Brazil. Data on treatment and postoperative survival were also evaluated.
METHODSThis was a single-center retrospective study based on data from an institutional database. At our center, the department of thoracic surgery not only has an internal database but also actively participates in databases of regional relevance-such as the São Paulo State Lung Cancer Registry-and of international relevance-such as the database of the Brazilian Society of Thoracic Surgery, which is linked to that of the European Society of Thoracic Surgeons, and the International Association for the Study of Lung Cancer (IASLC) database. The IASLC database, which is hosted on an international online platform, includes voluntary participation of various specialties, and gathers data on the clinical and epidemiological characteristics of lung cancer patients, as well as data on diagnosis, clinical/pathological staging, treatments, and survival. The information collected in various countries is used for assessing the prognostic value of factors currently used in the TNM classification and for assessing the use of new elements for potential inclusion in the staging system in the future. Ours is the only hospital in Brazil that is a contributor to the IASLC database,(14) and that collaboration allows greater representation of the Brazilian and Latin American populations in global studies that will result in periodic changes in lung cancer staging.
At our center, data entry is performed by physicians and nurses of the department of thoracic surgery, and entries are constantly audited by an experienced coordinator, who has authored studies in the area of data quality.(15,16) The data platform allows entry only of structured data and has security mechanisms that increase the accuracy and consistency of entries, ensuring that the data collected are of high quality.
In the present study, we used patient data from the IASLC database. We selected only cases of patients undergoing curative surgical resection of lung cancer between January of 2011 and April of 2018. Patients were followed until December of 2018. Patients without a pathology-confirmed diagnosis were excluded. Each entry in the database corresponds to a resected lung neoplasm, and more than one entry can exist for a given patient if they have had more than one lung neoplasm.
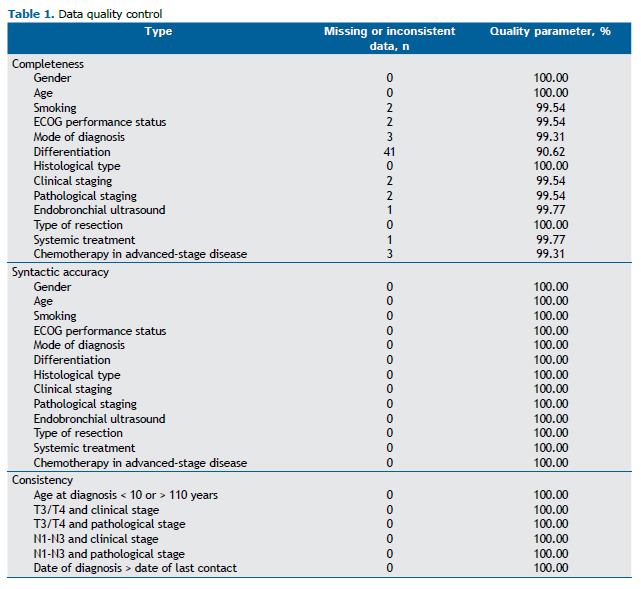
We initially analyzed data quality by using consolidated, indirect audit metrics, namely, completeness, accuracy, and consistency. The criteria for assessing consistency were age at diagnosis less than 10 years or greater than 100 years; date of diagnosis greater than the date of last contact (which directly affects the calculation of survival rates); and the presence of stages T3, T4, N1, N2, or N3 in patients classified as having clinical or pathological stage I disease. The staging criteria were adopted to assess the consistency of the data that depend directly on the health care professional performing the entries, given that only the basic data used to determine TNM staging are entered into the platform and staging is calculated manually. We used a minimum proportion of 80% as the standard of quality, as did the European Society of Thoracic Surgeons(17) when auditing its database.
We assessed demographic variables-gender, age at surgery, smoking, comorbidities, and ECOG performance status-tumor detection mode, histological type, clinical/pathological stage according to the eighth edition Lung Cancer Stage Classification,(18) type of surgical resection, 30-day mortality, and treatment with chemotherapy, radiotherapy, or both. To determine which variables affected survival, we performed Cox regression analysis of histological type, date of surgery (see below), and pathological stage. By using information gathered at the last contact, we determined overall survival and disease-free survival at 36 months. The analysis included only patients undergoing surgery prior to the end of December of 2015. We stratified the cases of resected lung cancer by period (on the basis of date of surgery): 2011-2014 and 2015-2018. We compared the two periods on the basis of the same data cited above, in order to determine whether there were differences over time in the variables of interest. We also compared the two periods in terms of unadjusted overall survival and overall survival adjusted for pathological stage.
Categorical variables were compared by using Pearson's chi-square test. For the continuous numeric variable (age), sample normality was tested with the Shapiro-Wilk test, and, subsequently, comparative analysis was performed by using the Mann-Whitney test because the sample was not normally distributed. Values of p < 0.05 were considered significant. From the postoperative follow-up data, we calculated overall survival and disease-free survival at 36 months for the entire study period, as well as for the 2011-2014 and 2015-2018 periods, using the Kaplan-Meier method and the log-rank test for comparisons. We used the STATA statistical software package, version 13 (Stata Corp, College Station, TX, USA).
The present study was evaluated and approved by the Research Ethics Committee of the University of São Paulo School of Medicine (Protocol no. 88741718.3.0000.0065). The requirement for written informed consent was waived because this was a retrospective study using medical record information from a database.
RESULTSBetween January of 2011 and April of 2018, there were a total of 442 cases of patients undergoing lung cancer, and those cases were subsequently entered into the IASLC database. We excluded 5 of those cases because of missing pathology results. After exclusions, the sample comprised 437 cases of lung cancer in 431 patients.
We initially assessed the quality of the data entered into the IASLC database, in order to determine whether it was feasible to analyze them in the present study (Table 1). No variables were excluded because of poor quality, given that all of the variables studied showed a completeness above 90%, an accuracy of 100%, and a consistency of 100%.
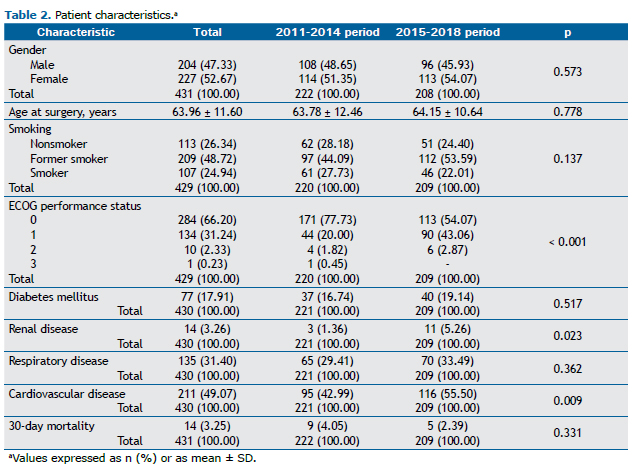
Analysis of the variables revealed higher proportions of female patients (52.67%) and of patients with a history of smoking (73.66%, of whom 48.72% were former smokers). The mean age at surgery was 63.96 ± 11.60 years. The most common comorbidities were cardiovascular disease (49.07%) and respiratory disease (31.40%). The 30-day mortality rate was 3.25%. The clinical and epidemiological data are detailed in Table 2.
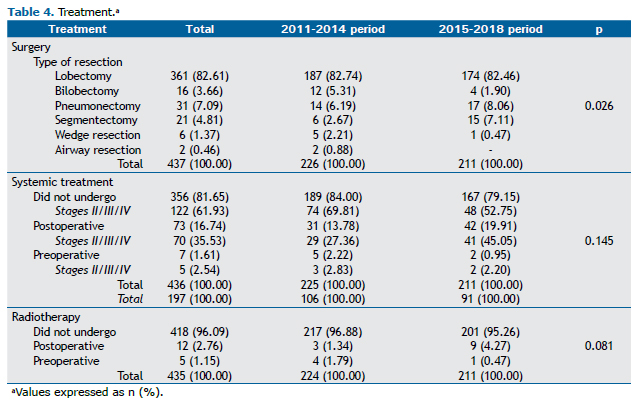
Most cases were diagnosed incidentally (60.14%), and the most common histological type was adenocarcinoma (55.84%). The distribution of clinical and pathological stages is described in Table 3, as are the other cancer-related data. Most patients underwent PET/CT for cancer staging (85.13%; Table 3). Of the surgical patients evaluated, 82.61% underwent lobectomy, 81.65% did not undergo chemotherapy, and 96.09% did not undergo radiotherapy (Table 4).

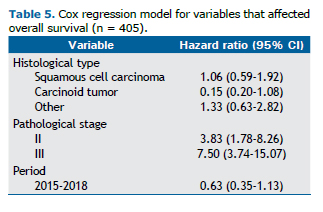
For the analysis of overall and disease-free survival at 36 months, we evaluated 285 and 263 patients, respectively, and the probability of survival at the end of the observation period was 79.4% (95% CI: 74.0-83.9%) and 75.1% (95% CI: 69.1-80.1%), respectively (Figure 1). Cox regression analysis revealed that only pathological stage was an independent factor associated with survival (Table 5).
After stratifying the cases by period, we found that 226 resections were performed in 222 patients (4 patients with two lesions) in the 2011-2014 period and that 211 resections were performed in 209 patients (2 patients with two lesions) in the 2015-2018 period.
Comparing the 2011-2014 and 2015-2018 periods, we observed an upward, although not statistically significant, trend in the number of female patients and a similar mean age (63.78 years vs. 64.15 years; p = 0.778). The proportion of former smokers increased (from 44.09% to 53.59%; p = 0.137). Although the proportion of patients with comorbidities was higher in the 2015-2018 period, mortality was lower, but not statistically significantly so (p = 0.331; Table 2).
In the 2011-2014 and 2015-2018 periods, most of the cases of lung cancer (52.47% and 68.25%, respectively) were diagnosed incidentally (p = 0.003). In both periods, adenocarcinoma was the most common histological type, followed by squamous cell carcinoma. However, the proportion of cases of adenocarcinoma increased from 52.21% to 59.72%, whereas the proportion of cases of squamous cell carcinoma decreased from 23.89% to 19.91% (p = 0.406 for both). In the 2015-2018 period, we observed an upward trend in the proportions of cases diagnosed at an earlier clinical stage (p = 0.002) and of cases diagnosed at an earlier pathological stage (p = 0.084; Table 3).
We observed that the proportion of surgical patients who underwent invasive staging by endobronchial ultrasound decreased from the 2011-2014 period to the 2015-2018 period (from 64.44% to 28.91%; p < 0.001), as did the proportion of those who underwent invasive staging by video-assisted mediastinoscopy (from 29.20% to 21.33%; p = 0.059). Data on the staging tests performed are presented in Table 3.
Although lobectomy was the most common type of surgical resection in both periods, we observed a significant increase in the proportion of segmentectomies (from 2.67% to 7.11%; p = 0.026). In addition, there was a trend toward an increase in the proportion of patients undergoing chemotherapy, especially adjuvant chemotherapy (from 13.78% to 19.91%; p = 0.145). After separating patients undergoing chemotherapy for pathological stage I disease from those undergoing chemotherapy for pathological stage II-IV disease (Table 4), we found that the increase was more marked among those diagnosed at a more advanced stage and that the number of patients undergoing adjuvant therapy nearly doubled in the 2015-2018 period. Treatment-related data are presented in Table 4.
The difference in overall survival between the periods lost statistical significance when adjusted for pathological stage (log-rank: p = 0.038 to p = 0.079; Figure 1).
 DISCUSSION
DISCUSSIONAfter an indirect audit, which confirmed the quality of the patient data, we found that the clinical and demographic characteristics were similar to those reported in recent studies conducted in Brazil,(5,10,11) except for the data related to overall and disease-free survival at 36 months, which were both above 75% in the present study. That difference is probably due to the characteristics of our study, in which we evaluated surgically curable patients (i.e., patients with earlier-stage disease). The similarity between overall and disease-free survival rates demonstrates the direct relationship between relapse/progression and mortality.
When comparing patients operated on before 2015 with those operated on after 2015, we found trends already seen worldwide, such as an increased incidence of lung cancer in women.(19,20) Brazil has been the country with the highest upward trend in the number of new lung cancer cases in women in the past 10 years.(21) An increased incidence of adenocarcinoma, together with a reduced incidence of squamous cell carcinoma,(22) was also observed in our patients. These trends are in agreement with those reported in other recent studies on the clinical and epidemiological profile of lung cancer in Brazil.(23,24) A progressive decrease in smoking rates in the country(25) was also found in the 2015-2018 period, during which there was a higher proportion of former smokers.
There is as yet no lung cancer screening program in Brazil, although there have been studies demonstrating the benefit of such a program, including the National Lung Screening Trial(26) and the Dutch-Belgian Lung Cancer Screening Trial,(27) as well as a study conducted in Brazil.(28) In the present study, less than 1% of patients were diagnosed by screening. In most cases, the diagnosis was made either incidentally, especially in patients operated on more recently, or on the basis of symptoms, forms of diagnosis that reduce the chances of early diagnosis and potentially curative surgical treatment. The increase in the number of incidental diagnoses may have occurred for one of two reasons: either patients underwent imaging studies more frequently and malignancy was a finding; or, more likely, the symptom-based diagnoses corresponded to more advanced disease for which surgical treatment is not indicated, which excluded the patients from the present analysis.
Earlier clinical and pathological stages were found to predominate among patients undergoing surgical resection between 2015 and 2018. Patients diagnosed at a more advanced stage, who thereby lost the benefit of surgical treatment, were probably referred for systemic therapy and therefore were not included in our analysis. Cox regression analysis revealed that only pathological stage had a significant impact on overall survival in our study, which, in a way, validated staging in our population. That variable was also a determinant of the difference in overall survival between the two periods analyzed, because, when the curves were adjusted for pathological stage, that difference lost statistical significance. Therefore, efficient patient selection was found to be the main factor associated with the increased survival observed in the 2015-2018 period. That factor is probably also related to the decrease in 30-day mortality in the 2015-2018 period, even among patients who were more severely ill, which is to be expected.(29)
Another factor that may have contributed to the decrease in mortality in the 2015-2018 period was improved surgical technique, given the increasing proportion of minimally invasive procedures, especially those performed by video-assisted thoracoscopy, as well as those performed by robotic surgery, which started being used in 2015.(30,31) Lobectomy was the most commonly performed surgical procedure, corresponding to more than 80% of the cases in the 2011-2014 and 2015-2018 periods. However, we observed a significant increase in segmentectomies in the 2015-2018 period, which is in line with a trend in the current surgical literature that underscores the benefits of sublobar resection as an alternative to lobectomy in patients with limited lung function, multifocal disease,(32) or early-stage disease.(33,34) Ongoing prospective studies are seeking to prove that overall survival is similar between anatomic sublobar resection and lobectomy,(35,36) and the similarity of postoperative morbidity and mortality between the two techniques has been proven.(36,37) We also observed a reduction in the number of wedge resections, which have limited oncological value.(38,39)
There were decreases in the use of invasive mediastinal staging by video-assisted mediastinoscopy and, especially, by endobronchial ultrasound, the decrease in the latter being statistically significant. These decreases are attributable to an increased rate of staging-based diagnosis of mediastinal lymph node involvement, with such patients being referred to the oncology department for systemic treatment alone after a diagnosis of locally advanced disease. Another factor that may explain these decreases is the increased incidence of earlier-stage cases that do not require invasive mediastinal staging.(40)
One advantage of the present study is the high reliability of the data analyzed. The fact that, at our center, data entry is performed by health care professionals committed to providing care and to maintaining the database, together with the fact that entries are constantly audited, certainly has a positive impact on the quality of the data analyzed, making the findings of the present study more reliable. Another advantage is the use of observed data rather than estimated data, thus providing an update on the lung cancer situation in Brazil and adding to the existing body of knowledge in terms of clinical and epidemiological data, as well as in terms of information on staging and treatment.
Because this was a study of surgically treated lung cancer patients, the evaluation of patients diagnosed at an advanced stage, who are still the majority among lung cancer patients in Brazil and were not included in the present study, could represent a limitation, potentially reducing the generalizability of the findings. The small number of patients is also a limitation because this was a single-center study. Another potential bias to be considered is the fact that, when comparing survival between the two periods, we found that the duration of follow-up was shorter in the 2015-2018 period.
We conclude that the clinical and epidemiological profile of lung cancer patients in Brazil has followed worldwide trends. We also found that advances in patient selection (priority being given to early-stage tumors), in surgical technique, and in perioperative care, as well as an increase in the number of segmentectomies, have led to lower surgical mortality rates. That decrease occurred despite the fact that patients who are more severely ill have undergone surgery with curative intent in recent years, underscoring the benefits of the changes implemented.
AUTHOR CONTRIBUTIONSRMT and PMPF: substantial contribution to the study design; data analysis and interpretation; drafting and revision of the manuscript; and approval of the final version to be published. MSS, PHCL, PBC, LMC, and LLL: data entry into the IASLC database; data collection and analysis and drafting and revision of the manuscript.
REFERENCES1. Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172(5):523-529. https://doi.org/10.1164/rccm.200504-531OE
2. World Health Organization. International Agency for Research on Cancer (IARC) [homepage on the Internet]. Lyon: IARC; c2018 [updated 2018 Dec 12; cited 2019 Mar 1]. New Global Cancer Data: GLOBOCAN. 2018. Available from: https://www.uicc.org/news/new-global-cancer-data-globocan-2018:~:text=Geneva%2C%20Switzerland%2C%2012%20September%202018,and%209.6%20million%20cancer%20deaths
3. Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Estimativa 2018: incidência de Câncer no Brasil. Rio de Janeiro: INCA; 2017.
4. Secretaria de Estado de Saúde de São Paulo. Fundação Oncocentro de São Paulo (FOSP). Estimativa de morbidade de câncer no estado de São Paulo: cânceres mais frequentes segundo ano de diagnóstico 2016. São Paulo: FOSP; 2016.
5. Araujo LH, Baldotto C, Castro G Jr, Katz A, Ferreira CG, Mathias C, et al. Lung cancer in Brazil. J Bras Pneumol. 2018;44(1):55-64. https://doi.org/10.1590/s1806-37562017000000135
6. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) [published correction appears in Lancet. 2015 Mar 14;385(9972):946]. Lancet. 2015;385(9972):977-1010. https://doi.org/10.1016/S0140-6736(14)62038-9
7. World Health Organization [homepage on the Internet]. Geneva: World Health Organization [cited 2018 Dec 2]. Global tuberculosis report 2018. [Adobe Acrobat document, 265p.]. Available from: http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1
8. Novaes FT, Cataneo DC, Ruiz Junior RL, Defaveri J, Michelin OC, Cataneo AJ. Lung cancer: histology, staging, treatment and survival. J Bras Pneumol. 2008;34(8):595-600. https://doi.org/10.1590/S1806-37132008000800009
9. Barros JA, Valladares G, Faria AR, Fugita EM, Ruiz AP, Vianna AG, et al. Early diagnosis of lung cancer: the great challenge. Epidemiological variables, clinical variables, staging and treatment. J Bras Pneumol. 2006;32(3):221-227. https://doi.org/10.1590/S1806-37132006000300008
10. Caires-Lima R, Takahashi TK, Mak MP, Roitberg FS, Teixeira CH, Mesquita CS, et al. Referral of lung cancer patients to specialized clinical oncology care: Instituto do Câncer do Estado de São Paulo 2010-2011. J Thorac Oncol. 2012;7(7):S111.
11. Araujo LH, Baldotto CS, Zukin M, Vieira FM, Victorino AP, Rocha VR, et al. Survival and prognostic factors in patients with non-small cell lung cancer treated in private health care. Rev Bras Epidemiol. 2014;17(4):1001-1014. https://doi.org/10.1590/1809-4503201400040017
12. Westphal FL, Lima LC, Andrade EO, Lima Netto JC, Silva AS, Carvalho BC. Characteristics of patients with lung cancer in the city of Manaus, Brazil. J Bras Pneumol. 2009;35(2):157-163. https://doi.org/10.1590/S1806-37132009000200009
13. Younes RN, Deutsch F, Badra C, Gross J, Haddad F, Deheinzelin D. Nonsmall cell lung cancer: evaluation of 737 consecutive patients in a single institution. Rev Hosp Clin Fac Med Sao Paulo. 2004;59(3):119-127. https://doi.org/10.1590/S0041-87812004000300005
14. Giroux DJ, Van Schil P, Asamura H, Rami-Porta R, Chansky K, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: A Renewed Call to Participation. J Thorac Oncol. 2018;13(6):801-809. https://doi.org/10.1016/j.jtho.2018.02.012
15. Lauricella LL. Análise da qualidade de uma base de dados a partir da implementação do Registro Paulista de Tratamento Cirúrgico de Câncer de Pulmão [thesis]. São Paulo: Universidade de São Paulo; 2017. Available from: http://www.incor.usp.br/sites/incor2013/docs/LETICIA_LEONE_LAURICELLA_1.pdf
16. Lauricella LL, Costa PB, Salati M, Pego-Fernandes PM, Terra RM. Measurement of the Inter-Rater Reliability Rate Is Mandatory for Improving the Quality of a Medical Database: Experience with the Paulista Lung Cancer Registry. J Am Coll Surg. 2018;226(6):1128-1136. https://doi.org/10.1016/j.jamcollsurg.2018.03.006
17. Salati M, Brunelli A, Dahan M, Rocco G, Van Raemdonck DE, Varela G. Task-independent metrics to assess the data quality of medical registries using the European Society of Thoracic Surgeons (ESTS) Database. Eur J Cardiothorac Surg. 2011;40(1):91-98. https://doi.org/10.1016/j.ejcts.2010.11.004
18. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151(1):193-203. https://doi.org/10.1016/j.chest.2016.10.010
19. Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol. 2016;11(10):1653-1671. https://doi.org/10.1016/j.jtho.2016.05.021
20. Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32(4):605-644. https://doi.org/10.1016/j.ccm.2011.09.001
21. Wong MCS, Lao XQ, Ho KF, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep. 2017;7(1):14300. https://doi.org/10.1038/s41598-017-14513-7
22. Gabrielson E. Worldwide trends in lung cancer pathology. Respirology. 2006;11(5):533-538. https://doi.org/10.1111/j.1440-1843.2006.00909.x
23. Tsukazan MT, Vigo Á, Silva VD, Barrios CH, Rios JO, Pinto JA. Lung cancer: changes in histology, gender, and age over the last 30 years in Brazil. J Bras Pneumol. 2017;43(5):363-367. https://doi.org/10.1590/s1806-37562016000000339
24. Costa GJ, de Mello MJG, Ferreira CG, Bergmann A, Thuler LCS. Increased incidence, morbidity and mortality rates for lung cancer in women in Brazil between 2000 and 2014: An analysis of three types of sources of secondary data. Lung Cancer. 2018;125:77-85. https://doi.org/10.1016/j.lungcan.2018.09.005
25. Portes LH, Machado CV, Turci SRB, Figueiredo VC, Cavalcante TM, Silva VLDCE. Tobacco Control Policies in Brazil: a 30-year assessment. Cien Saude Colet. 2018;23(6):1837-1848. https://doi.org/10.1590/1413-81232018236.05202018
26. The National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. https://doi.org/10.1056/NEJMoa1102873
27. The ASCO Post [homepage on the Internet]. Huntington, NY: Harborside [updated 2018 Sep 26; cited 2019 Mar 1]. WCLC 2018: NELSON Study: CT Screening for Early Lung Cancer Reduces Lung Cancer Mortality. Available from: http://www.ascopost.com/News/59300
28. dos Santos RS, Franceschini JP, Chate RC, Ghefter MC, Kay F, Trajano ALC, et al. Do Current Lung Cancer Screening Guidelines Apply for Populations With High Prevalence of Granulomatous Disease? Results From the First Brazilian Lung Cancer Screening Trial (BRELT1). Ann Thorac Surg. 2016;101(2):481-488. https://doi.org/10.1016/j.athoracsur.2015.07.013
29. Seder CW, Wright CD, Chang AC, Han JM, McDonald D, Kozower BD. The Society of Thoracic Surgeons General Thoracic Surgery Database Update on Outcomes and Quality. Ann Thorac Surg. 2016;101(5):1646-1654. https://doi.org/10.1016/j.athoracsur.2016.02.099
30. Terra RM, Araujo PH, Lauricella LL, Campos JR, Costa HF, Pego-Fernandes PM. Robotic pulmonary lobectomy for lung cancer treatment: program implementation and initial experience. J Bras Pneumol. 2016;42(3):185-190. https://doi.org/10.1590/S1806-37562015000000212
31. Terra RM, Bibas BJ, Haddad R, Milanez-de-Campos JR, Nabuco-de-Araujo PHX, Teixeira-Lima CE, et al. Robotic thoracic surgery for non-small cell lung cancer: initial experience in Brazil. J Bras Pneumol. 2019;46(1):e20190003. https://doi.org/10.1590/1806-3713/e20190003
32. Charloux A, Quoix E. Lung segmentectomy: does it offer a real functional benefit over lobectomy?. Eur Respir Rev. 2017;26(146):170079. https://doi.org/10.1183/16000617.0079-2017
33. Sagawa M, Oizumi H, Suzuki H, Uramoto H, Usuda K, Sakurada A, et al. A prospective 5-year follow-up study after limited resection for lung cancer with ground-glass opacity. Eur J Cardiothorac Surg. 2018;53(4):849-856. https://doi.org/10.1093/ejcts/ezx418
34. Hennon M, Landreneau RJ. Role of Segmentectomy in Treatment of Early-Stage Non-Small Cell Lung Cancer. Ann Surg Oncol. 2018;25(1):59-63. https://doi.org/10.1245/s10434-017-5787-5
35. Nakamura K, Saji H, Nakajima R, Okada M, Asamura H, Shibata T, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol. 2010;40(3):271-274. https://doi.org/10.1093/jjco/hyp156
36. Altorki NK, Wang X, Wigle D, Gu L, Darling G, Ashrafi AS, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med. 2018;6(12):915-924. https://doi.org/10.1016/S2213-2600(18)30411-9
37. Suzuki K, Saji H, Aokage K, Watanabe S, Okada M, Mizusawa J, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg. 2019;158(3):895-907. https://doi.org/10.1016/j.jtcvs.2019.03.090
38. Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest. 2014;145(1):66-71. https://doi.org/10.1378/chest.13-1094
39. Landreneau RJ, Schuchert MJ. Is segmentectomy the future? J Thorac Dis. 2019;11(1):308-318. https://doi.org/10.21037/jtd.2018.12.67
40. De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45(5):787-798. https://doi.org/10.1093/ejcts/ezu028







