ABSTRACT
Objective: To evaluate the performance of the No-Apnea score, a simplified screening instrument for obstructive sleep apnea (OSA), by gender. Methods: This was a cross-sectional study including adults undergoing full polysomnography. The No-Apnea model comprises two items (neck circumference and age) with a total score of 0 to 9. The severity of OSA was categorized, on the basis of the apnea-hypopnea index, as any (≥ 5 events/h), moderate-to-severe (≥ 15 events/h), or severe (≥ 30 events/h). The performance of the No-Apnea instrument was assessed by determining the area under the (ROC) curve (AUC) and by constructing contingency tables. Results: We evaluated a total of 6,606 adults (53.8% men). For categorizing the level of OSA severity, the No-Apnea score had a sensitivity of 83.9-93.0% and a specificity of 57.3-35.2%. At all OSA severity levels, the No-Apnea score exhibited higher sensitivity and lower specificity in men than in women. The No-Apnea score proved to be an appropriate screening model for patients in general or when separated by gender or severity of OSA (AUC > 0.7 for all). The discriminatory power of the No-Apnea score to predict any, moderate-to-severe, and severe OSA was similar between genders (p = 0.109, p = 0.698, and p = 0.094, respectively). Conclusions: In a sample of adults referred to the sleep laboratory, there was no significant difference between men and women in terms of the discriminatory power of the No-Apnea instrument in for screening for OSA severity.
Keywords:
Sleep apnea, obstructive/diagnosis; Polysomnography; Sex; Surveys and questionnaires.
RESUMO
Objetivo: Avaliar o desempenho do escore No-Apnea, um instrumento simplificado para a triagem da apneia obstrutiva do sono (AOS), relacionado ao gênero. Métodos: Estudo transversal incluindo indivíduos adultos submetidos à polissonografia completa. O No-Apnea é um modelo contendo dois itens (circunferência do pescoço e idade) com uma pontuação total de 0 a 9. A gravidade da AOS foi baseada no índice de apneia-hipopneia ≥ 5 eventos/h (qualquer AOS), ≥ 15 eventos/h (AOS moderada/grave) e ≥ 30 eventos/h (AOS grave). O desempenho do No-Apnea foi avaliado pela área sob a curva (ASC) ROC e tabelas de contingência. Resultados: No total, 6.606 adultos (53,8% homens) foram avaliados. No geral, para a triagem dos diferentes níveis de gravidade de AOS, o No-Apnea teve sensibilidade variando de 83,9% a 93,0% e especificidade variando de 57,3% a 35,2%. Em todos os níveis de gravidade da AOS, o No-Apnea exibiu maior sensibilidade e menor especificidade nos homens que nas mulheres. O No-Apnea mostrou ser um adequado modelo de triagem para os pacientes em geral ou quando separados por gênero ou gravidade da AOS (ASC > 0,7 para todos). A capacidade discriminatória do No-Apnea em predizer qualquer AOS, AOS moderada/grave e AOS grave foi semelhante entre os sexos (p = 0,109, p = 0,698 e p = 0,094, respectivamente). Conclusões: Em uma amostra de indivíduos adultos encaminhados para laboratório do sono, o No-Apnea apresentou discriminação similar para a triagem de AOS de acordo com sua gravidade em mulheres e homens.
Palavras-chave:
Apneia obstrutiva do sono/diagnóstico; Polissonografia; Sexo; Inquéritos e questionários.
INTRODUCTIONObstructive sleep apnea (OSA) is a sleep disorder characterized by recurrent episodes of upper airway obstruction, resulting in intermittent hypoxemia, disruptions in sleep, and cardiovascular problems.(1-3) The prevalence of OSA has increased considerably in recent years,(4-6) possibly because of the aging population and the global obesity epidemic. One recent study reported that the overall prevalence of OSA was 32.8% in the city of São Paulo, Brazil.(6)
It is common for sleep laboratories around the world to have a long list of individuals with suspected OSA waiting to get tested. To date, the gold standard test for diagnosing OSA is full polysomnography (PSG). However, it is an expensive test that is not widely available, especially in regions with limited economic resources. Therefore, a screening instrument offering a simplified or home-based diagnostic method can be useful for stratifying patients.
The No-Apnea score is an instrument that comprises only two objective parameters-neck circumference (NC) and age-with a final score ranging from 0 to 9 (a score ≥ 3 indicates a high risk for OSA).(7) ) In the No-Apnea derivation cohort, the area under the ROC curve (AUC) was 0.784, 0.758, and 0.754 for screening for any, moderate-to-severe, and severe OSA, respectively. In fact, despite the simplicity of the No-Apnea score, when compared with two other previously validated models, its discriminatory power showed no statistically significant difference.(7)
As for the clinical history, men with OSA usually display typical symptoms, such as snoring and observed apnea, whereas women often report atypical symptoms, such as insomnia, morning headache, and fatigue.(8-12) In comparison with male patients, female patients typically are older, are more obese, and have more comorbidities-such as hypertension and diabetes mellitus.(10-13) However, NC tends to be greater in men than in women.(14) Based on the polysomnographic findings, women have a lower prevalence of OSA and show evidence of lower quality of sleep than do men.(8-12) As we can see, significant differences can be found between men and women with OSA, not only in the prevalence of the disease but also in the clinical phenotypes associated with it. However, despite the several gender-related differences consistently reported in the clinical presentation and polysomnographic findings of the condition,(8-14) analyses of the performance of OSA screening instruments by gender are surprisingly rare. In view of the above, the main objective of the present study was to evaluate the predictive performance and discriminatory power of the No-Apnea score, a simplified model for screening for OSA, by gender.
METHODSThis was a prospective study, carried out between January of 2017 and March of 2019, with recruitment of individuals who were referred for sleep assessments by their attending physicians. The inclusion criteria were being ≥ 18 years of age and having a suspected sleep disorder. Patients who had previously been diagnosed with OSA were excluded, as were those who were diagnosed through the use of a portable or home monitoring device, those for whom the clinical data were incomplete, and those in whom the PSG was technically inadequate. The study protocol was in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of the Federal University of Rio de Janeiro (Reference no. 1.764.165). All participants gave written informed consent. If the same patient was submitted to more than one PSG, the test with the longest total sleep time was selected for analysis.
The clinical characteristics included gender, age, body mass index (BMI), NC, self-reported comorbidities (smoking, hypertension, and diabetes mellitus), and sleep-related complaints (snoring, observed apnea, nocturnal choking, and morning headache). Patients also completed five instruments, all of which have been validated in the literature: the No-Apnea score(7); the Snoring, Tiredness, Observed apnea, and high blood Pressure (STOP) and Snoring, Tiredness, Observed apnea, high blood Pressure, Body mass index, Age, Neck circumference, and Gender (STOP-Bang) questionnaires(15); the Neck circumference, obesity, Snoring, Age, and Sex (NoSAS) score(16); and the Epworth Sleepiness Scale (ESS).(17) All of those the instruments have also been validated for use with the Brazilian population.(7,16,18,19) The screening instruments were applied by the PSG technicians immediately prior to the sleep test. The BMI was calculated as the weight in kilograms divided by the height in meters squared (kg/m2), and the NC (in cm) was systematically measured with a measuring tape, as follows(7): patients were asked to remain erect; and the NC was measured with the upper edge of the measuring tape just below the laryngeal prominence.
Screening instrumentsThe No-Apnea model evaluates two objective parameters (NC and age), scored as follows: NC of 37.0-39.9 cm = 1; NC of 40.0-42.9 cm = 3; NC of ≥ 43.0 cm = 6; age of 35-44 years = 1; age of 45-54 years = 2; and age of ≥ 55 years = 3. The scores given to each variable are summed, generating a final score ranging from 0 to 9 (a score ≥ 3 indicates a high risk for OSA).(7)
The STOP and STOP-Bang questionnaires(15,18) consist of four and eight yes/no questions, respectively. Each affirmative answer gets a score of 1. The STOP questionnaire contains questions about loud snoring, tiredness, observed apnea, and hypertension (the total score ranging from 0 to 4), whereas the STOP-Bang questionnaire uses those same parameters plus BMI > 35 kg/m2, age > 50 years, NC > 40 cm, and male gender (the total score ranging from 0 to 8). The STOP and STOP-Bang questionnaires use a score of ≥ 2 and ≥ 3, respectively, to identify individuals at risk for OSA.
The NoSAS instrument is scored as follows: an NC > 40 cm gets a score of 4; BMIs of 25-29 kg/m2 and ≥ 30 kg/m2 get scores of 3 and 5, respectively; snoring gets a score of 2; age > 55 years gets a score of 4; and being a male gets a score of 2. The score ranges from 0 to 17 and is considered positive when a patient gets a score ≥ 8.(16)
The ESS is an eight-item instrument that assesses the likelihood of a patient falling asleep in various contexts. Each question is answered on a scale from 0 (never dozes off) to 3 (high chance of dozing off), with a final score ranging from 0 to 24 (a score ≥ 11 indicates excessive daytime sleepiness).(17)
Sleep studiesAll polysomnographic evaluations were performed on the same type of device (EMBLA S7000; Embla Systems Inc., Broomfield, CO, USA), at the same sleep center in the city of Rio de Janeiro, Brazil. The recordings consisted of continuous monitoring by electroencephalography, electro-oculography, chin/leg electromyography, and electrocardiography, as well as of airflow, respiratory effort (with chest and abdominal belts), SpO2 (by pulse oximetry), snoring (with a tracheal microphone), and body position (with position sensors). Two pulmonologists performed the manual reading of the exams, as recommended by the American Academy of Sleep Medicine.(20) Both were blinded to the results obtained with the screening instruments. Apnea was defined as a ≥ 90% drop in the baseline airflow value for at least ten seconds was classified as apnea, whereas hypopnea was defined as a ≥ 30% drop for at least 10 seconds accompanied by a ≥ 3% drop in oxygen saturation or a microarousal.(20) The level of OSA severity was classified, on the basis of the apnea-hypopnea index (AHI), as any (AHI ≥ 5 events/h), moderate-to-severe (AHI ≥ 15 events/h), or severe (AHI ≥ 30 events/h).
Statistical analysisThe data were analyzed with the IBM SPSS Statistics software package, version 21.0 (IBM Corporation, Armonk, NY, USA) and are expressed as means ± standard deviation (for numerical variables) or as absolute and relative frequencies (for categorical variables). We used the chi-square test to compare dichotomous variables, whereas we used the Student's t-test and ANOVA to compare numerical variables. The predictive value of the No-Apnea score was assessed on the basis of its discriminatory power and by contingency tables. The discriminatory power was estimated on the basis of the AUC, which can vary from 0.5 (no discrimination) to 1.0 (perfect discrimination). (21) An AUC > 0.7 was considered clinically significant.(22) The discriminatory power was compared by using a methodology previously described.(23) Sensitivity, specificity, positive predictive value, and negative predictive value were calculated from the contingency tables, and all values are expressed with their respective 95% CIs. A two-tailed p value < 0.05 was considered statistically significant.
RESULTSOf a total of 6,820 consecutive individuals who were referred for OSA workup, 214 (3.1%) were excluded on the basis of the study criteria. Therefore, 6,606 patients were enrolled for further analysis: 3,054 (46.2%) were female and 3,552 (53.8%) were male. In comparison with the male patients, the female patients were older, had a higher BMI, and had a lower NC (p < 0.001 for all), as shown in Table 1. Diabetes mellitus was more prevalent in women than in men (p < 0.001). All sleep parameters evaluated were statistically different between genders, except for rapid eye movement sleep (p = 0.334). The mean AHI was higher in men than in women (37.2 ± 29.6 events/h vs. 18.9 ± 22.3 events/h; p < 0.001), whereas the SpO2 nadir was lower in men than in women (80.0 ± 9.8% vs. 83.5 ± 8.8%; p < 0.001), suggesting that OSA was more severe in men than in women. The prevalence of any, moderate-to-severe, and severe OSA was statistically higher in men than in women-88.5% vs. 67.9%, 71.1% vs. 41.9%, and 51.2% vs. 20.9%, respectively (p < 0.001 for all). In addition, the likelihood of having any, moderate-to-severe, and severe OSA was statistically higher in men than in women-OR = 3.626 (95% CI: 3.190-4.121), OR = 3.403 (95% CI: 3.073-3.769), and OR = 3.966 (95% CI: 3.555-4.424), respectively.
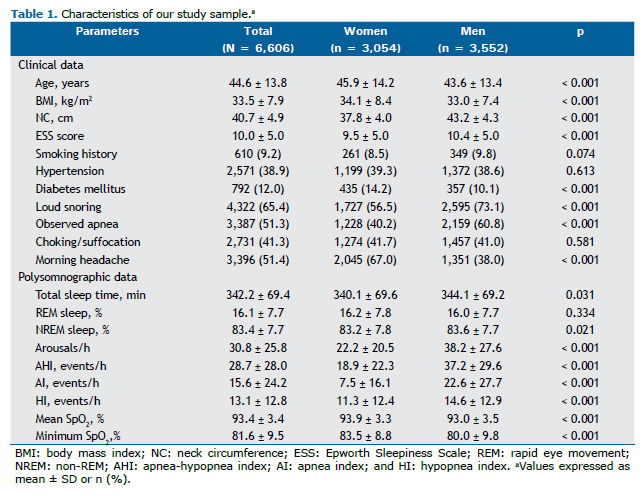
The mean No-Apnea score was significantly lower in women than in men (3.2 ± 2.2 vs. 5.5 ± 2.3; p < 0.001). Overall, 75.3% of the patients were classified as being at high risk for OSA (No-Apnea score ≥ 3), the proportion of high-risk individuals being higher among the men than among the women (88.0% vs. 60.4%; p < 0.001). The proportional distribution of women and men by No-Apnea score is shown in Figure 1.
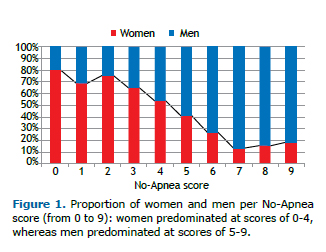
For both genders, an increase in the No-Apnea score from 0 to 9 led to an increase in the prevalence of OSA-that of any OSA increased from 27.6% to 94.4% in women and from 53.5% to 96.6% in men; that of moderate-to-severe OSA increased from 10.8% to 76.7% in women and from 25.4% to 85.2% in men; and that of severe OSA went from 2.0% to 63.3% in women and from 12.7% to 68.3% in men. Similarly, with the progressive increase in the No-Apnea score, there was a trend toward a linear increase in the mean AHI (Figure 2): from 5.1 ± 8.6 events/h to 42.8 ± 28.8 events/h in women (p < 0.001); and from 12.1 ± 16.1 events/h to 46.8 ± 27.1 events/h in men (p < 0.001).
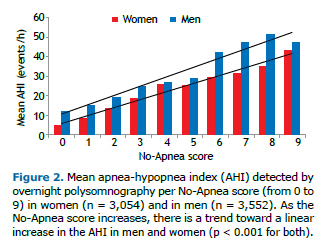
Table 2 shows the performance of the No-Apnea score by gender. Overall, for screening different levels of OSA severity, the sensitivity of the No-Apnea model ranged from 83.9% to 93.0%, whereas its specificity ranged from 57.3% to 35.2%. Regardless of the level of severity, the No-Apnea model had higher sensitivity and lower specificity in men than in women.
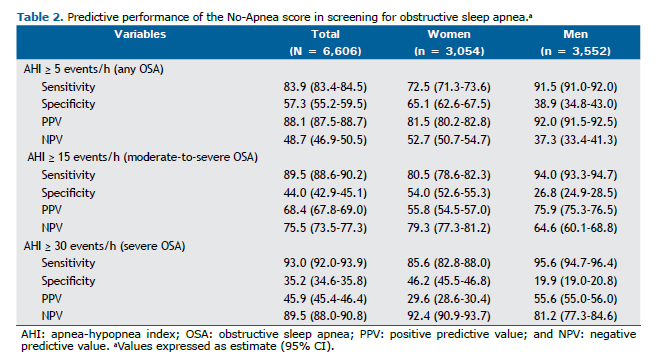
Table 3 shows the discriminatory power calculated for each of the five screening instruments: the No-Apnea score, the STOP questionnaire, the STOP-Bang questionnaire, the NoSAS score, and the ESS. The No-Apnea proved to be a useful screening tool for all patients included in the study and for patients dichotomized by gender (AUC > 0.7 for all OSA severity levels). In women, the AUC obtained ranged from 0.719 (95% CI: 0.701-0.737) to 0.741 (95% CI: 0.721-0.760), whereas in men, it ranged from 0.702 (95% CI: 0.685-0.720) to 0.763 (95% CI: 0.738-0.788). The discriminatory power of the No-Apnea score for any, moderate-to-severe, and severe OSA was comparable between the genders (p = 0.109, p = 0.698, and p = 0.094, respectively). The other models also had similar performances for both genders, except for the ESS, which performed better in men than in women for any, moderate-to-severe, and severe OSA (p = 0.007, p = 0.009, and p = 0.015, respectively).
 DISCUSSION
DISCUSSIONThe main finding of our study was that, in adult individuals who were referred to a sleep laboratory, the No-Apnea score can be a useful screening tool for OSA. The instrument showed appropriate predictive performance and discriminatory power for the purpose of screening for OSA at all levels of severity and in both genders.
An advantage of using OSA screening instruments like the No-Apnea score is the possibility of appropriately referring high-risk patients for evaluation with portable diagnostic methods, thereby reducing the long waiting lists at sleep laboratories.(24,25) In addition, because the No-Apnea score comprises only objective variables, it can be used in individuals who sleep alone and whose subjective sleep information is not always easily available.
In the present study, we found several clinical and polysomnographic differences between genders that have already been extensively reported in the literature. (8-14) We found a predominance of OSA in men, which is in keeping with the findings of population-based studies(6) and studies conducted in sleep laboratories,(18) albeit different that what has been reported in studies of patients in the preoperative period of bariatric surgery(26) or of patients with insomnia.(27) One previous study showed that the prevalence of OSA was lower in women than in men, despite the fact that the women in the sample had higher BMIs and were older.(28) The following factors have been implicated in the gender-related difference in OSA(29-31): hormonal influences and menopause (in women); craniofacial structure; and upper airway length.
There are several OSA screening instruments available, and their performance may vary depending on the tests used to diagnose OSA, the type of population evaluated, and the AHI cutoff used.(32) It is possibly more important that screening tests for diseases like OSA have high sensitivity than that they have high specificity, especially in a population with a high pretest probability.(32,33) Preeminent among the several OSA screening models described in the literature are the Berlin questionnaire,(34) the STOP-Bang questionnaire,(15) and the NoSAS score.(16) Although the gender-related differences in the symptoms and prevalence of OSA are well established, few studies have effectively evaluated whether there are also gender-specific differences in the performance of the screening instruments.
A study assessing the applicability of the fractional exhaled nitric oxide test as a screening method for OSA found that the No-Apnea score was a useful screening tool for any, moderate-to-severe, and severe OSA, for which the AUC reported was 0.786, 0.713, and 0.717, respectively.(35) A subsequent study, involving a cohort of morbidly obese patients, found that the No-Apnea score had appropriate discriminatory power for screening for OSA.(36) The authors found no gender-specific differences in performance for the screening for any (p = 0.973) and moderate-to-severe OSA (p = 0.817), although they did find the score to perform better in screening for severe OSA in women than in men (p = 0.033).(36) The No-Apnea score has also been validated in a cohort of patients with insomnia, showing an appropriate predictive performance.(37) As previously reported,(7,36,37) its discriminatory power is similar to that of other instruments with positive evaluations in the literature, such as the STOP-Bang questionnaire(15) and the NoSAS score.(16)
In a study of 502 patients (465 men and 37 women) who underwent portable monitoring sleep studies, a STOP-Bang score ≥ 3 predicted an AHI ≥ 5 events/h with an AUC of 0.72.(38) Sensitivity and specificity rates were calculated separately for men and women but achieved similar results, the sensitivity being 98.8% and 100.0%, respectively, whereas the specificity was 4.0% and 0.0%, respectively. However, that study(38) had significant limitations that are worth mentioning: few women were included; all participants were evaluated with unsupervised sleep studies; and no comparisons were made between the men and the women in terms of the AUC.
Another study, involving 1,426 individuals undergoing full PSG, found that observed apnea and snoring were reported more often in men, whereas the presence of tiredness and hypertension was similar between genders.(39) However, gender-specific AUCs have not been reported for the STOP-Bang questionnaire. A study involving 251 patients (76% women) undergoing preoperative evaluation for bariatric surgery applied four different instruments (the ESS, the Fatigue Severity Scale, the STOP-Bang questionnaire, and the NoSAS score) and found that, except for the ESS, all of the instruments performed better in women than in men.(40)
A study of 403 women and 532 men found that the performance of the STOP-Bang questionnaire in screening for OSA in women was influenced by the BMI, whereas NC seemed to be more relevant in the screening of men.(41) That study also showed that the STOP-Bang questionnaire had extremely low specificity in men: 11.9% for any OSA (AHI ≥ 5 events/h), 7.9% for moderate-to-severe OSA (AHI ≥ 15 events/h), and 7.0% for severe OSA (AHI ≥ 30 events/h). In our study, the No-Apnea score also showed low specificity in men: 38.9% for any OSA (AHI ≥ 5 events/h), 26.8% for moderate-to-severe OSA (AHI ≥ 15 events/h), and 19.9% for severe OSA (AHI ≥ 30 events/h). However, our values were higher than those found for the STOP-Bang questionnaire.(41)
The present study has some limitations. The sample was composed of patients referred to a single sleep laboratory (i.e., preselected individuals with a high pretest probability), which could limit the generalizability of our findings. In addition, it did not include many individuals of other ethnicities, who could have different anthropometric characteristics.
In conclusion, the present study, involving adult individuals who were referred to a sleep laboratory, identified several clinical and polysomnographic differences between genders. Nevertheless, the No-Apnea score showed appropriate performance in screening for suspected OSA across all severity levels. Because the prevalence of OSA increases in parallel with increases in the No-Apnea score, this model can be used to aid in classifying risk in individuals referred to sleep laboratories, regardless of gender.
REFERENCES1. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479-504. https://doi.org/10.5664/jcsm.6506
2. Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J. 2016;47(4):1162-1169. https://doi.org/10.1183/13993003.01618-2015
3. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The Rational Clinical Examination systematic review. JAMA. 2013;310(7):731-741. https://doi.org/10.1001/jama.2013.276185
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. https://doi.org/10.1093/aje/kws342
5. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. https://doi.org/10.1016/S2213-2600(15)00043-0
6. Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441-446. https://doi.org/10.1016/j.sleep.2009.10.005
7. Duarte RLM, Rabahi MF, Magalhães-da-Silveira FJ, de Oliveira-E-Sá TS, Mello FCQ, Gozal D. Simplifying the Screening of Obstructive Sleep Apnea With a 2-Item Model, No-Apnea: A Cross-Sectional Study. J Clin Sleep Med. 2018;14(7):1097-1107. https://doi.org/10.5664/jcsm.7202
8. Yamakoshi S, Kasai T, Tomita Y, Takaya H, Kasagi S, Kawabata M, et al. Comparison of clinical features and polysomnographic findings between men and women with sleep apnea. J Thorac Dis. 2016;8(1):145-151. https://doi.org/10.3978/j.issn.2072-1439.2016.01.49
9. Bozkurt MK, Oy A, Aydin D, Bilen SH, Ertürk IO, Saydam L, et al. Gender differences in polysomnographic findings in Turkish patients with obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2008;265(7):821-824. https://doi.org/10.1007/s00405-007-0554-z
10. Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath. 2018;22(1):241-249. https://doi.org/10.1007/s11325-017-1482-9
11. Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, Lavie P. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaire. Sleep. 2007;30(3):312-319. https://doi.org/10.1093/sleep/30.3.312
12. Nigro CA, Dibur E, Borsini E, Malnis S, Ernst G, Bledel I, et al. The influence of gender on symptoms associated with obstructive sleep apnea. Sleep Breath. 2018;22(3):683-693. https://doi.org/10.1007/s11325-017-1612-4
13. O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161(5):1465-1472. https://doi.org/10.1164/ajrccm.161.5.9904121
14. Dancey DR, Hanly PJ, Soong C, Lee B, Shepard J Jr, Hoffstein V. Gender differences in sleep apnea: the role of neck circumference. Chest. 2003;123(5):1544-1550. https://doi.org/10.1378/chest.123.5.1544
15. Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812-821. https://doi.org/10.1097/ALN.0b013e31816d83e4
16. Marti-Soler H, Hirotsu C, Marques-Vidal P, Vollenweider P, Waeber G, Preisig M, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742-748. https://doi.org/10.1016/S2213-2600(16)30075-3
17. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545. https://doi.org/10.1093/sleep/14.6.540
18. Duarte RLM, Fonseca LBM, Magalhães-da-Silveira FJ, Silveira EAD, Rabahi MF. Validation of the STOP-Bang questionnaire as a means of screening for obstructive sleep apnea in adults in Brazil. J Bras Pneumol. 2017;43(6):456-463. https://doi.org/10.1590/s1806-37562017000000139
19. Bertolazi AN, Fagondes SC, Hoff LS, Pedro VD, Menna Barreto SS, Johns MW. Portuguese-language version of the Epworth sleepiness scale: validation for use in Brazil. J Bras Pneumol. 2009;35(9):877-883. https://doi.org/10.1590/S1806-37132009000900009
20. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597-619. https://doi.org/10.5664/jcsm.2172
21. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128-138. https://doi.org/10.1097/EDE.0b013e3181c30fb2
22. Cowan DC, Allardice G, Macfarlane D, Ramsay D, Ambler H, Banham S, et al. Predicting sleep disordered breathing in outpatients with suspected OSA. BMJ Open. 2014;4(4):e004519. https://doi.org/10.1136/bmjopen-2013-004519
23. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839-843. https://doi.org/10.1148/radiology.148.3.6878708
24. Andrade L, Paiva T. Ambulatory Versus Laboratory Polysomnography in Obstructive Sleep Apnea: Comparative Assessment of Quality, Clinical Efficacy, Treatment Compliance, and Quality of Life. J Clin Sleep Med. 2018;14(8):1323-1331. https://doi.org/10.5664/jcsm.7264
25. Rosen IM, Kirsch DB, Carden KA, Malhotra RK, Ramar K, Aurora RN, et al. Clinical Use of a Home Sleep Apnea Test: An Updated American Academy of Sleep Medicine Position Statement. J Clin Sleep Med. 2018;14(12):2075-2077. https://doi.org/10.5664/jcsm.7540
26. Duarte RL, Magalhães-da-Silveira FJ. Factors predictive of obstructive sleep apnea in patients undergoing pre-operative evaluation for bariatric surgery and referred to a sleep laboratory for polysomnography. J Bras Pneumol. 2015;41(5):440-448. https://doi.org/10.1590/S1806-37132015000000027
27. Krell SB, Kapur VK. Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005;9(3):104-110. https://doi.org/10.1007/s11325-005-0026-x
28. Subramanian S, Jayaraman G, Majid H, Aguilar R, Surani S. Influence of gender and anthropometric measures on severity of obstructive sleep apnea. Sleep Breath. 2012;16(4):1091-1095. https://doi.org/10.1007/s11325-011-0607-9
29. Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181-1185. https://doi.org/10.1164/rccm.200209-1055OC
30. Mohsenin V. Gender differences in the expression of sleep-disordered breathing : role of upper airway dimensions. Chest. 2001;120(5):1442-1447. https://doi.org/10.1378/chest.120.5.1442
31. Segal Y, Malhotra A, Pillar G. Upper airway length may be associated with the severity of obstructive sleep apnea syndrome. Sleep Breath. 2008;12(4):311-316. https://doi.org/10.1007/s11325-008-0191-9
32. Ramachandran SK, Josephs LA. A meta-analysis of clinical screening tests for obstructive sleep apnea. Anesthesiology. 2009;110(4):928-939. https://doi.org/10.1097/ALN.0b013e31819c47b6
33. Senaratna CV, Perret JL, Matheson MC, Lodge CJ, Lowe AJ, Cassim R, et al. Validity of the Berlin questionnaire in detecting obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med Rev. 2017;36:116-124. https://doi.org/10.1016/j.smrv.2017.04.001
34. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485-491. https://doi.org/10.7326/0003-4819-131-7-199910050-00002
35. Duarte RLM, Rabahi MF, Oliveira-E-Sá TS, Magalhães-da-Silveira FJ, Mello FCQ, Gozal D. Fractional Exhaled Nitric Oxide Measurements and Screening of Obstructive Sleep Apnea in a Sleep-Laboratory Setting: A Cross-Sectional Study. Lung. 2019;197(2):131-137. https://doi.org/10.1007/s00408-018-0190-y
36. Duarte RLM, Mello FCQ, Magalhães-da-Silveira FJ, Oliveira-E-Sá TS, Rabahi MF, Gozal D. Comparative performance of screening instruments for obstructive sleep apnea in morbidly obese patients referred to a sleep laboratory: a prospective cross-sectional study. Sleep Breath. 2019;23(4):1123-1132. https://doi.org/10.1007/s11325-019-01791-w
37. Duarte RLM, Magalhães-da-Silveira FJ, Oliveira-E-Sá TS, Rabahi MF, Mello FCQ, Gozal D. Predicting Obstructive Sleep Apnea in Patients with Insomnia: A Comparative Study with Four Screening Instruments. Lung. 2019;197(4):451-458. https://doi.org/10.1007/s00408-019-00232-5
38. Doshi V, Walia R, Jones K, Aston CE, Awab A. STOP-BANG questionnaire as a screening tool for diagnosis of obstructive sleep apnea by unattended portable monitoring sleep study. Springerplus. 2015;4:795. https://doi.org/10.1186/s40064-015-1588-0
39. Farney RJ, Walker BS, Farney RM, Snow GL, Walker JM. The STOP-Bang equivalent model and prediction of severity of obstructive sleep apnea: relation to polysomnographic measurements of the apnea/hypopnea index. J Clin Sleep Med. 2011;7(5):459-65B. https://doi.org/10.5664/JCSM.1306
40. Horvath CM, Jossen J, Kröll D, Nett PC, Baty F, Brill AK, et al. Prevalence and Prediction of Obstructive Sleep Apnea Prior to Bariatric Surgery-Gender-Specific Performance of Four Sleep Questionnaires. Obes Surg. 2018;28(9):2720-2726. https://doi.org/10.1007/s11695-018-3222-z
41. Mou J, Pflugeisen BM, Crick BA, Amoroso PJ, Harmon KT, Tarnoczy SF, et al. The dis-criminative power of STOP-Bang as a screening tool for suspected obstructive sleep apnea in clinically referred patients: considering gender differences. Sleep Breath. 2019;23(1):65-75. https://doi.org/10.1007/s11325-018-1658-y








