ABSTRACT
Objective: To evaluate the frequency of and factors associated with indeterminate interferon-gamma release assay (IGRA) results in people living with HIV/AIDS (PLWHA). Methods: We tested 81 PLWHA in the central-west region of Brazil, using the tuberculin skin test and an IGRA. Information on sociodemographic and clinical variables was gathered through the use of questionnaires and from medical records. The association of those variables with indeterminate results was analyzed by calculating the adjusted ORs in a multivariate logistic regression model. Concordance was evaluated by determining the kappa statistic. Results: Among the 81 patients evaluated, the tuberculin skin test results were positive in 18 (22.2%) of the patients, and the IGRA results were positive in 10 (12.3%), with a kappa of 0.62. The IGRA results were indeterminate in 22 (27.1%) of the patients (95% CI: 17.8-38.1%). The odds of obtaining indeterminate results were significantly higher in smokers (adjusted OR = 6.0; 95% CI: 1.4-26.7) and in samples stored for less than 35 days (adjusted OR = 14.0; 95% CI: 3.1-64.2). Patients with advanced immunosuppression (CD4+ T-cell count < 200 cells/mm3) were at a higher risk for indeterminate results (OR adjusted for smoking and inadequate duration of sample storage = 4.7; 95% CI: 0.91-24.0), although the difference was not significant. Conclusions: The high prevalence of indeterminate results can be a major limitation for the routine use of IGRAs in PLWHA. The need to repeat the test increases its costs and should be taken into account in cost-effectiveness studies. The processing of samples can significantly alter the results.
Keywords:
Interferon-gamma release tests; Interferon-gamma; Tuberculosis; HIV; Latent tuberculosis; Tuberculin test.
RESUMO
Objetivo: Avaliar a frequência de resultados indeterminados de um interferon-gamma release assay (IGRA, ensaio de liberação de interferon-gama) e os fatores relacionados com esses resultados em pessoas vivendo com HIV/AIDS (PVHA). Métodos: Foram avaliadas 81 PVHA na região Centro-Oeste do Brasil, por meio do teste tuberculínico e de um IGRA. Informações a respeito de variáveis sociodemográficas e clínicas foram obtidas por meio de questionários e prontuários médicos. A relação entre essas variáveis e os resultados indeterminados foi avaliada por meio do cálculo da OR ajustada em um modelo de regressão logística multivariada. A concordância foi avaliada por meio do coeficiente kappa. Resultados: Os resultados do teste tuberculínico e do IGRA foram positivos em 18 (22,2%) e 10 (12,3%), respectivamente, dos 81 pacientes avaliados (κ = 0,62). O resultado do IGRA foi indeterminado em 22 (27,1%) dos pacientes (IC95%: 17,8-38,1%). A chance de resultados indeterminados foi significativamente maior em fumantes (OR ajustada = 6,0; IC95%: 1,4-26,7) e em amostras armazenadas durante menos de 35 dias (OR ajustada = 14,0; IC95%: 3,1-64,2). Pacientes com imunossupressão avançada (contagem de células T CD4+ < 200 células/mm3) apresentaram maior risco de resultados indeterminados (OR ajustada para tabagismo e tempo inadequado de armazenamento das amostras = 4,7; IC95%: 0,91-24,0), embora a diferença não tenha sido significativa. Conclusões: A alta prevalência de resultados indeterminados pode ser um grande obstáculo ao uso rotineiro de IGRAs em PVHA. A necessidade de repetir o teste aumenta seu custo e deve ser levada em conta em estudos da relação entre custo e eficácia. O processamento das amostras pode alterar significativamente os resultados.
Palavras-chave:
Testes de liberação de interferon-gama; Interferon gama; Tuberculose; HIV; Tuberculose latente; Teste tuberculínico.
INTRODUÇÃOEm pessoas vivendo com HIV/AIDS (PVHA), o tratamento da infecção tuberculosa latente (ITBL) é crucial para reduzir a morbidade e a mortalidade.(1) Portanto, a identificação de ITBL e seu tratamento adequado em PVHA são prioridades. Atualmente, há duas classes de testes para detectar ITBL(2): o teste tuberculínico (TT) e os interferon-gamma (IFN-γ) release assays (IGRAs, ensaios de liberação de IFN-γ). Dois IGRAs disponíveis comercialmente foram amplamente estudados: o ensaio QuantiFERON-TB Gold In-Tube (QFT-GIT; Cellestis, Carnegie, Austrália) e o ensaio T-SPOT.TB (Oxford Immunotec, Abingdon, Reino Unido). Os IGRAs substituíram o TT ou se juntaram a ele em muitos países de alta renda.(2) No entanto, a Organização Mundial da Saúde não recomenda esses testes para a detecção de ITBL em países de baixa e média renda. Tanto o TT como os IGRAs apresentam menor sensibilidade em PVHA, pois ambos avaliam a resposta de células T a antígenos micobacterianos.(3) Embora tenham sido relatadas altas taxas de resultados indeterminados do ensaio QFT-GIT em países com alta carga de HIV, essas taxas podem variar de acordo com a região geográfica e a gravidade da imunossupressão.(4,5) Na presença de resultados indeterminados, recomenda-se que se repita o teste.(6)
A descontinuação temporária recente do mais usado PPD (RT23; Statens Serum Institut, Copenhague, Dinamarca) resultou em escassez de tuberculina no mercado, o que impediu a identificação de ITBL em PVHA em diversos países. Substituir o TT pelo ensaio QFT-GIT poderia ser uma opção, mesmo em países de baixa renda. O objetivo do presente estudo foi avaliar a frequência de resultados indeterminados do ensaio QFT-GIT e os fatores relacionados com esses resultados em uma amostra de PVHA.
MÉTODOSDe janeiro a dezembro de 2011, foi realizado um estudo transversal na Clínica de Doenças Infecciosas da Universidade Federal de Mato Grosso do Sul. Mato Grosso do Sul é um estado com incidência moderada de tuberculose (38,8/100.000 habitantes), localizado na região Centro-Oeste do Brasil. As Diretrizes Nacionais de Tuberculose recomendam que todas as PVHA sejam submetidas ao TT no mínimo a cada seis meses.(7) Neste estudo, foram incluídos indivíduos infectados pelo HIV com idade ≥ 18 anos, todos os quais assinaram um termo de consentimento livre e esclarecido para a realização de testes adicionais com o QFT-GIT. Os pacientes com tuberculose ativa foram excluídos, assim como o foram aqueles que estavam recebendo tratamento para ITBL. O estudo foi aprovado pelo Comitê de Ética em Pesquisa da Universidade Federal de Mato Grosso do Sul (Protocolo n. 1060/2008).
As informações foram obtidas por meio de um questionário e de prontuários médicos. Cada TT foi realizado com duas unidades de PPD RT23 (Statens Serum Institut) na face anterior do antebraço esquerdo. Após 48-72 h, uma enfermeira treinada mediu a enduração. Uma enduração ≥ 5 mm de diâmetro foi considerada indicativa de reação positiva.(7) O ensaio QFT-GIT foi realizado em conformidade com o protocolo do fabricante. Em resumo, amostras de sangue total foram colhidas diretamente em tubos heparinizados de 1 ml contendo antígenos de Mycobacterium tuberculosis (alvo antigênico secretório precoce 6, proteína de filtrado de cultura 10 e TB7.7); dextrose e PHA (controle positivo); ou sem antígenos (controle negativo). Os tubos foram incubados durante 24 h a 37°C e centrifugados a 2.000 g durante 10 min. Em seguida, o sobrenadante do soro foi colhido. A mediana de tempo decorrido da coleta do sangue até a incubação foi de 144 min (variação: 10-294 min). Todos os sobrenadantes foram armazenados a −70°C por até 8 semanas, até que se realizasse ELISA para determinar a quantidade de IFN-γ secretado. O ensaio foi realizado em lotes de 24-44 amostras do mesmo lote. O software fornecido pelo fabricante foi usado para analisar os resultados. O resultado do ensaio QFT-GIT era considerado positivo se o nível de IFN-γ após a estimulação com antígenos de M. tuberculosis menos o controle negativo fosse ≥ 0,35 UI/ml e 25% maior do que a concentração de IFN-γ na amostra não estimulada de controle; o resultado era considerado negativo se o nível de IFN-γ fosse < 0,35 UI/ml. O resultado era considerado indeterminado se a produção de IFN-γ na amostra não estimulada fosse ≥ 8,0 UI/ml ou a PHA menos a concentração de IFN-γ na amostra não estimulada fosse < 0,5 UI/ml. Em casos em que o resultado do primeiro ensaio QFT-GIT foi indeterminado, não se realizou um segundo ensaio; a decisão clínica baseou-se no TT.(7) A concordância entre o ensaio QFT-GIT e o TT foi avaliada por meio do coeficiente kappa. A concordância era considerada excelente se o coeficiente kappa fosse > 0,75, razoável se fosse = 0,4-0,75 e ruim se fosse < 0,4. Para o cálculo da concordância, foram incluídos apenas os resultados positivos e negativos. As proporções foram comparadas por meio de OR ajustada (ORa) e seu intervalo de confiança de 95% em um modelo de regressão multivariada. As análises foram realizadas por meio do programa SPSS Statistics, versão 20.0 (IBM Corporation, Armonk, NY, EUA).
RESULTADOSOitenta e uma PVHA foram incluídas no estudo. A mediana de idade foi de 41 anos (variação: 34-48 anos); a mediana da contagem de células T CD4+ foi de 422 células/mm3 [intervalo interquartil (II): 221-646 células/mm3]. Os resultados do TT e do ensaio QFT-GIT foram positivos em 18 (22,2%) e 10 (12,3%), respectivamente, dos 81 participantes. O resultado do ensaio QFT-GIT foi indeterminado em 22 (27,1%) dos participantes (IC95%: 17,8-38,1%). A concordância entre os dois testes (entre os resultados válidos) foi classificada em razoável (κ = 0,62; IC95%: 0,37-0,87), principalmente em virtude de um maior número de resultados negativos do TT (Tabela 1).
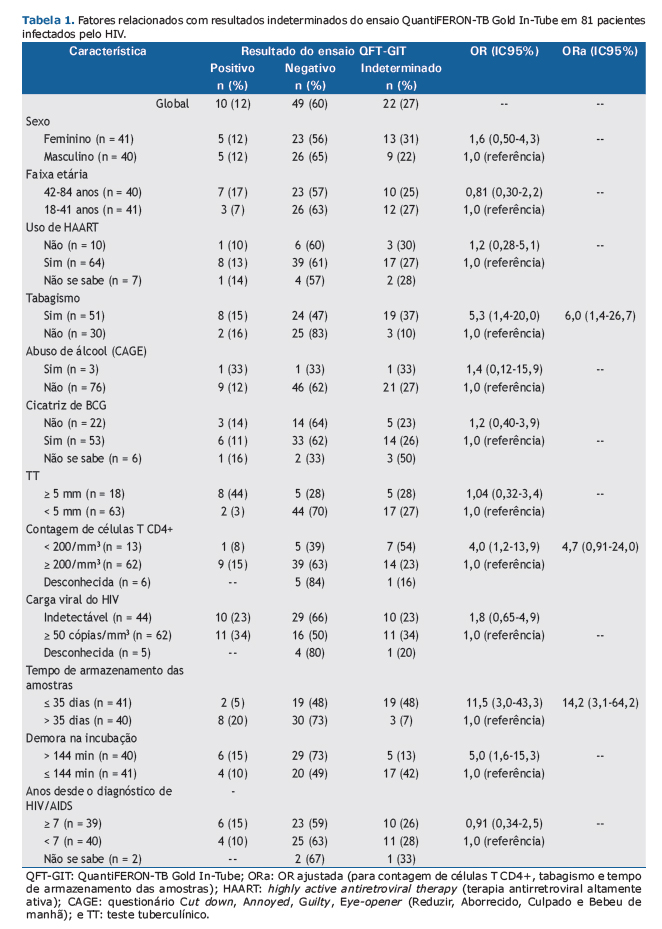
Nos participantes cujos resultados foram indeterminados, a mediana da contagem de células T CD4+ foi de 329 células/mm3 (II: 156-575 células/mm3), em comparação com 494 células/mm3 (II: 235-696 células/mm3) naqueles cujos resultados foram válidos (p = 0,237). A chance de resultado indeterminado foi 6,0 vezes maior em fumantes, 14,2 vezes maior em casos em que o tempo de armazenamento das amostras foi < 35 dias e 4,7 vezes em participantes com imunossupressão avançada (contagem de células T CD4+ < 200 células/mm3), embora esta última não tenha sido estatisticamente significativa, mesmo após ajuste para tempo de armazenamento e tabagismo (Tabela 1).
DISCUSSÃORelatou-se que a frequência de resultados indeterminados do ensaio QFT-GIT varia de 0% a 19,8%. (8-12) Muitas variáveis relacionam-se com resultados indeterminados: infecção pelo HIV,(9) imunossupressão avançada,(9) vacinação prévia com BCG,(10) terapia imunossupressora,(11) doenças subjacentes,(11) estar acamado,(8) hipoalbuminemia(8) e tuberculose ativa. (13) Aspectos técnicos também resultam em um aumento significativo da proporção de resultados indeterminados(6,9,11): defeitos de fabricação; erros analíticos; demora na incubação ou no processamento; técnica incorreta de agitação dos tubos e volume sanguíneo inadequado. Em nosso estudo, a proporção de resultados indeterminados do ensaio QFT-GIT foi maior que a relatada na literatura. Diferentemente de relatos anteriores, essa proporção não se relacionou significativamente nem com a contagem de células T CD4+ nem com a vacinação com BCG, embora poucos participantes tenham apresentado contagem de células T CD4+ inferior a 200 células/mm3, o que reduziu o poder de nossa análise. Por outro lado, o tabagismo e o tempo menor de armazenamento do soro antes do processamento para ELISA aumentaram sobremaneira a chance de resultados indeterminados. O tabagismo pode induzir imunossupressão por meio de mecanismos que não a redução da contagem de células T CD4+, tais como a redução da resposta proliferativa a mitógenos.(14) Não sabemos da existência de nenhum relato de que o tempo de armazenamento no refrigerador afete os resultados do ensaio QFT-GIT, e não podemos oferecer nenhuma explicação razoável para nosso achado de que um tempo menor de armazenamento do soro aumentou a chance de resultados indeterminados. Independentemente do motivo dos resultados indeterminados, é recomendado realizar um segundo teste quando o resultado do primeiro é indeterminado, e a repetição de testes aumentará significativamente os custos laboratoriais.(5) Embora nossa amostra tenha sido pequena, acreditamos que não tenha havido um viés de seleção.
Em resumo, o presente estudo mostrou que uma alta proporção de ensaios QFT-GIT apresentou resultados indeterminados, o que representa um grande obstáculo ao uso de ensaios desse tipo em PVHA no Brasil. Esse achado tem implicações relevantes para a relação entre custo e eficácia e a viabilidade do uso de ensaios QFT-GIT em países de baixa e média renda.
REFERÊNCIAS1. Bucher HC, Griffith LE, Guyatt GH, Sudre P, Naef M, Sendi P, et al. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS. 1999;13(4):501-7. https://doi.org/10.1097/00002030-199903110-00009
2. Kunst H. Diagnosis of latent tuberculosis infection: The potential role of new technologies. Respir Med. 2015;100(12):2098-106. https://doi.org/10.1016/j.rmed.2006.02.032#
3. Khawcharoenporn T, Apisarnthanarak A, Phetsuksiri B, Rudeeaneksin J, Srisungngam S, Mundy LM. Tuberculin skin test and QuantiFERON-TB Gold In-tube Test for latent tuberculosis in Thai HIV-infected adults. Respirology. 2015;20(2):340-7. https://doi.org/10.1111/resp.12442
4. Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27(1):3-20. https://doi.org/10.1128/CMR.00034-13
5. Trajman A, Steffen RE, Menzies D. Interferon-Gamma Release Assays versus Tuberculin Skin Testing for the Diagnosis of Latent Tuberculosis Infection: An Overview of the Evidence. Pulm Med. 2013;2013:601737. https://doi.org/10.1155/2013/601737
6. Couturier MR, Myatt R, Dorn D, Yang DT, Pitstick N. Defective antigen tubes generate false-positive QuantiFERON tuberculosis test results. Clin Infect Dis. 2014;59(11):1649-50. https://doi.org/10.1093/cid/ciu644
7. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Programa Nacional de Controle da Tuberculose. Manual de recomendações para o controle da tuberculose no Brasil. Brasília: Ministério da Saúde; 2011.
8. Yun JW, Chung HS, Koh WJ, Chung DR, Kim YJ, Kang ES. Significant reduction in rate of indeterminate results of the QuantiFERON-TB Gold In-Tube test by shortening incubation delay. J Clin Microbiol. 2014;52(1):90-4. https://doi.org/10.1128/JCM.01547-13
9. Oni T, Gideon HP, Bangani N, Tsekela R, Seldon R, Wood K, et al. Risk factors associated with indeterminate gamma interferon responses in the assessment of latent tuberculosis infection in a high-incidence environment. Clin Vaccine Immunol. 2012;19(8):1243-7. https://doi.org/10.1128/CVI.00166-12
10. Herrera V, Yeh E, Murphy K, Parsonnet J, Banaei N. Immediate incubation reduces indeterminate results for QuantiFERON-TB Gold in-tube assay. J Clin Microbiol. 2010;48(8):2672-6. https://doi.org/10.1128/JCM.00482-10
11. Cattamanchi A, Smith R, Steingart KR, Metcalfe J, Date A, Coleman C, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2011;56(3):230-8. https://doi.org/10.1097/QAI.0b013e31820b07ab
12. Kussen GM, Dalla-Costa LM, Rossoni A, Raboni SM. Interferon-gamma release assay versus tuberculin skin test for latent tuberculosis infection among HIV patients in Brazil. Braz J Infect Dis. 2016;20(1):69-75. https://doi.org/10.1016/j.bjid.2015.10.007
13. Sauzullo I, Mengoni F, Ermocida A, Massetti AP, D'Agostino C, Russo G, et al. Interferon-γ release assay in HIV-infected patients with active tuberculosis: impact of antituberculous drugs on host immune response. New Microbiol. 2014;37(2):153-61.
14. Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2(5):372-7. https://doi.org/10.1038/nri803




