ABSTRACT
Objective: To evaluate the effects of exposure to emissions from sugar cane burning on inflammatory mechanisms in tissues of the trachea and lung parenchyma in Wistar rats after different periods of exposure. Methods: This was an experimental open randomized study. The animals were divided into four groups: a control group (CG) underwent standard laboratory conditions, and three experimental groups were exposed to emissions from sugar cane burning over different periods of time, in days-1 (EG1), 7 (EG7), and 21 (EG21). After euthanasia with 200 mg/kg of ketamine/xylazine, fragments of trachea and lung were collected and fixed in 10% formalin. Histological analyses were performed with H&E and picrosirius red staining. Results: No inflammatory infiltrates were found in the tissues of CG rats. The histological examination of tissues of the trachea and lung parenchyma revealed that the inflammatory process was significantly more intense in EG7 than in the CG (p < 0.05 and p < 0.01, respectively). In comparison with the CG and EG1, angiogenesis in the lung parenchyma and collagen deposition in tracheal tissues were significantly greater only in EG21 (p < 0.001 and p < 0.01, respectively). Conclusions: In this sample, emissions from sugar cane burning induced acute focal and diffuse inflammation in the lamina propria of tracheal tissues, with no loss of ciliated epithelial tissue. In the lung parenchyma of the animals in the experimental groups, there was interstitial and alveolar edema, together with polymorphonuclear cell infiltrates.
Keywords:
Saccharum; Smoke; Inflammation; Respiratory system.
RESUMO
Objetivo: Avaliar os efeitos da exposição à fumaça da queima da cana-de-açúcar sobre mecanismos inflamatórios em tecidos de traqueia e de parênquima pulmonar de ratos Wistar após diferentes períodos de exposição. Métodos: Estudo experimental, randomizado, não cego. Os animais foram divididos em quatro grupos: controle (GC), sob condições padrão de laboratório e os demais expostos à fumaça da queima da cana-de-açúcar por diferentes períodos: em 1 (GE1), 7 (GE7) e 21 (GE21) dias. Após a eutanásia com 200 mg/kg de ketamina/xilazina, foram coletados fragmentos de traqueia e pulmão e fixadas em formol 10%. Análises histológicas foram realizadas com coloração com H&E e picrosírius. Resultados: Não houve infiltrado inflamatório nos tecidos no GC. O processo inflamatório na análise histológica de tecidos de traqueia e de parênquima pulmonar foi significativamente mais intenso no GE7 quando comparado ao GC (p < 0,05 e p < 0,01, respectivamente). Em comparação com os grupos GC e GE1, apenas no GE21 foi observada angiogênese significativa no parênquima pulmonar e aumento significativo de depósitos de colágeno em tecido de traqueia (p < 0,001 e p < 0,01, respectivamente). Conclusões: Nesta amostra, a fumaça da queima de cana-de-açúcar induziu processo inflamatório focal, difuso e agudo em tecidos de traqueia na lâmina própria, sem perda do tecido epitelial ciliado. Houve presença de edemas intersticiais e alveolares e infiltrados de células polimorfonucleares no parênquima pulmonar nos animais dos grupos experimentais.
Palavras-chave:
Saccharum; Fumaça; Inflamação; Sistema respiratório.
INTRODUÇÃOA cana-de-açúcar, Saccharum officinarum, é amplamente cultivada no Brasil para a produção de etanol e açúcar, sendo essencial à economia do país.(1,2) Sua colheita, quando realizada de forma manual, é precedida por queima dos canaviais para retirar as folhas secas, facilitar o corte e reduzir o risco de animais peçonhentos.(3-7)
A combustão da cana-de-açúcar libera grande quantidade de material particulado (MP), além de gases, como ozônio, monóxido de carbono, óxido nítrico, óxido de enxofre, formaldeídos, benzopireno e hidrocarbonetos policíclicos aromáticos,(6-8) que contribuem para a poluição atmosférica e repercutem negativamente na saúde humana.(2,9,10)
O MP e os compostos tóxicos gerados na queima são nocivos ao trato respiratório, pois ao serem inalados e depositarem-se nas vias aéreas inferiores, são fagocitados por macrófagos alveolares, que liberam citocinas citotóxicas gerando inflamação.(4,8) O estresse causado pela fumaça pode desencadear uma série de reações celulares com o objetivo de alcançar um estado de estabilidade; porém, quando esse estresse é crônico, provoca lesão celular irreversível.(11) O MP grosso presente na fumaça lesiona as vias áreas superiores por serem diretamente expostas ao meio externo, enquanto o MP fino se acumula nos brônquios e bronquíolos levando a lesão permanente e fibrose.(12,13) Estruturas alveolares são susceptíveis às respostas de natureza inflamatória e podem gerar reações patológicas com consequências obstrutivas e restritivas,(11,12,14-16) em geral associadas ao processo de remodelamento tissular.(14)
Embora conhecidos os riscos gerados pela exposição à fumaça, como a do tabaco ou da queima de combustíveis fósseis, há escassez de trabalhos correlacionados ao tema. Assim, o objetivo da presente investigação foi utilizar um modelo experimental para avaliar os efeitos da exposição à fumaça resultante da queima da cana-de-açúcar sobre mecanismos inflamatórios no tecido traqueal e no parênquima pulmonar, em diferentes períodos de exposição.
MÉTODOSEstudo experimental, randomizado e não cego. No estudo, 28 ratos machos Wistar, pesando 250-300 g, foram mantidos sob temperaturas variando de 25-28°C, com ciclo claro/escuro de 12 h, em caixas forradas com serragem e com livre acesso a ração e água filtrada. Os animais foram divididos em quatro grupos: grupo controle (GC), com 4 animais, que ficou sob condições padrão de laboratório por 24 h, e três grupos exposição, com 8 animais cada, correspondendo aos grupos de animais que foram submetidos à inalação de fumaça da queima da palha da cana-de-açúcar por períodos consecutivos de 1, 7 e 21 dias, respectivamente, denominados GE1, GE7 e GE21. Para esse fim, confeccionou-se uma câmara de combustão com um dispositivo portátil com exaustor de ar, onde foram queimadas 200 g de palha da cana-de-açúcar, gerando um fluxo contínuo de fumaça que foi canalizada através de uma tubulação para as gaiolas dos ratos dos grupos experimentais por um período de 2 h, diariamente e no mesmo horário (Figura 1).

Os animais foram eutanasiados com a administração i.p de ketamina/xilazina 5% diluídas em 2 ml de solução salina fisiológica. Após esse procedimento, a traqueia foi exposta e canulada e procederam-se uma laparotomia para o afastamento dos órgãos e a secção da artéria aorta abdominal e da veia cava inferior. A traqueia foi então ocluída por fio de sutura, a fim de se manter os pulmões em capacidade residual funcional. Realizou-se uma toracotomia transesternal, por meio do diafragma, para a retirada das traqueias e dos pulmões. Os órgãos coletados foram lavados com solução salina fisiológica para o exame macroscópico e fixados em formol tamponado a 10%.
Fragmentos teciduais obtidos do material foram processados de forma convencional e incluídos em parafina para a confecção de lâminas em secções de 5 μm. Os cortes foram corados pelas técnicas de H&E e picrosírius para a análise em um microscópio de luz (BX51; Olympus Optical, Tóquio, Japão) com aumento 100×. Para a obtenção de fotomicrografias, utilizou-se uma câmera digital (C-7070; Olympus. Com o intuito de se realizar uma análise microscópica semiquantitativa, adotaram-se critérios representativos para as alterações histológicas encontradas: leve, moderada e intensa.
Para a análise morfométrica foram obtidas três fotomicrografias de campos não coincidentes dos tecidos estudados. Nos cortes de traqueia, selecionaram-se áreas contendo cartilagem hialina, lâmina própria e epitélio ciliado. No parênquima pulmonar, foram avaliados bronquíolos, alvéolos e vasos sanguíneos. A quantificação da área de massa nuclear, em pixels, para a avaliação do processo inflamatório foi realizada utilizando-se o programa Adobe Photoshop CS5 (Adobe Systems Inc.; San Jose, CA, EUA). Os depósitos de colágeno foram quantificados pela tonalidade vermelha atribuída à coloração picrosírius, que corresponde à presença de colágeno do tipo I. A angiogênese foi analisada por técnica de contagem do número de vasos por quadrante em uma fotomicrografia panorâmica pelo programa BioEstat 5.3.
Uma análise estatística foi realizada pelo teste de normalidade de Shapiro-Wilk. Após a confirmação da normalidade, utilizou-se ANOVA com teste post hoc de Tukey. Nos demais casos, utilizaram-se o teste de Kruskal-Wallis e o teste post hoc de Dunn. Os resultados foram expressos como médias e desvios-padrão dos grupos e em disposições gráficas do tipo box plot. O nível de significância adotado foi de 5%.
O estudo foi aprovado pelo Comitê de Ética e Pesquisa no Uso de Animais de Laboratório da Faculdade Adventista da Bahia, sob o no. 013/2014.
RESULTADOSA análise macroscópica da traqueia não revelou alterações na coloração e na integridade tecidual em nenhum dos grupos. Os pulmões dos ratos submetidos à fumaça de GE7 e GE21 apresentaram alterações macroscópicas focais e difusas nos diferentes lóbulos, relacionadas à coloração e à textura tecidual.
As avaliações histológicas de cada grupo revelaram o seguinte:
No GC, não foram reveladas a presença de infiltrados inflamatórios ou alterações estruturais teciduais nas traqueias dos animais (Figura 2). O parênquima pulmonar não apresentou alterações alveolares, septais e bronquiolares, estando compatível com o padrão de normalidade (Figura 3).


No GE1, ocorreu um infiltrado inflamatório de leve intensidade na traqueia em 87,5% dos casos. No entanto, em 12,5% das amostras analisadas, a resposta foi intensa e com predomínio focal (Figura 2). No parênquima pulmonar, 75% dos animais apresentaram processo inflamatório, sendo 50% de células polimorfonucleares, com predomínio da forma difusa sobre a focal. Os infiltrados foram encontrados em áreas perivasculares, que variaram de leve a moderada, em regiões peribronquiolares. Foram evidenciados, também, edema intersticial leve e ausência de depósitos de colágeno (Figura 3).
No GE7, os animais apresentaram infiltrados inflamatórios variando a intensidade de leve (em 62,5%) moderada (em 50,0%) e intensa (em 25,0%) no tecido traqueal (Figura 2). Foi possível visualizar o início da formação de depósitos de colágeno pelo aumento da reação acidófila na traqueia através da coloração picrosírius (Figura 2). Na análise microscópica do parênquima pulmonar, o processo inflamatório ocorreu em 87,5% dos animais, e houve infiltrado de células polimorfonucleares em 62,5% dos casos. Em 75% dos animais, ocorreu processo inflamatório difuso, com intensidade variando de leve a intensa. Ocorreu um padrão de infiltrados perivasculares em 62,5% dos espécimes. Depósitos de colágeno nas regiões perivasculares e peribronquiolares também foram constatados (Figura 3).
No GE21, foi evidenciado infiltrado inflamatório difuso em 75% dos casos, com intensidade de grau leve (25%) a moderado (50%). Não houve casos de infiltrado inflamatório intenso (Figura 2). Na coloração com picrosírius, observou-se intensa acidofilia, indicando presença de colágeno (Figura 2). Pela análise histológica do parênquima pulmonar, foi possível observar infiltrados inflamatórios difusos, com predomínio de células mononucleares, com intensidade variando de leve a intensa em 100% dos animais. Houve inflamação perivascular e peribronquiolar, de leve intensidade, em 50% e 25% dos casos, respectivamente. Observaram-se necrose em 37,5% e angiogênese em 100% dos casos (p < 0,001) quando o grupo foi comparado com o GC e GE1, além de se visualizar áreas de depósitos de colágeno perivasculares e peribronquiolares, também em região alveolar (Figura 3).
A análise morfométrica e estatística (Figura 4) demonstrou presença de processo inflamatório no tecido traqueal nos grupos expostos. As médias da área de massa nuclear, em pixels, foram de 379,78 ± 105,65, no GC; 650,36 ± 147,74, no GE1; 899,18 ± 183,65, no GE7; e 751,96 ± 143,64, no GE21. A resposta inflamatória intensa aconteceu no GE7 na comparação com o GC (p < 0,05). Os dados da análise morfométrica são complementares aos achados da análise semiquantitativa.

A morfometria pela coloração de picrosírius revelou ausência de depósitos de colágeno no GC e um leve aumento progressivo na acidofilia em GE1 e GE7; porém, sem significância estatística. Contudo, foi detectada uma maior quantificação de depósitos de colágeno no tecido de traqueia no GE21, quando comparado a GC e GE1 (p < 0,01 para ambos; Figura 4).
A morfometria do parênquima pulmonar foi avaliada pela média da área de massa nuclear, em pixels. No GC, essa foi de 893,13 ± 51,89, compatível com a normalidade. Em GE1 iniciou-se um aumento da celularidade de polimorfonucleares e mononucleares, com área de massa nuclear de 1.373,66 ± 155,43, e atingindo 2.280,98 ± 744,80 no GE7 (p < 0,01 na comparação intergrupo). Porém, houve uma diminuição da área de massa nuclear para 1.251,31 ± 231,75 no GE21, sugestivo de reparo tecidual (Figura 5).
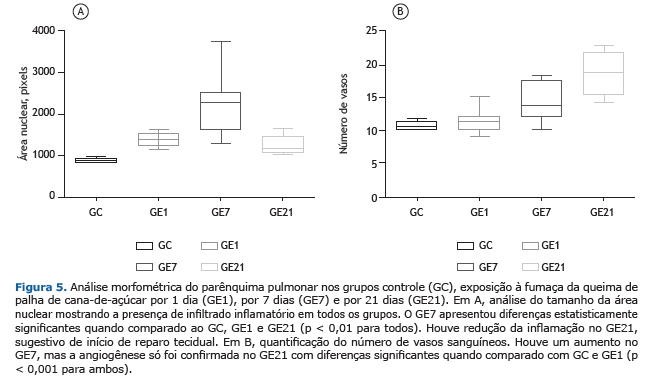
A morfometria da angiogênese mostrou que a mesma não ocorreu em GC, GE1 e GE7. Já no GE21 foi detectada a presença de angiogênese em 100% dos casos (Figura 5).
DISCUSSÃOEvidenciou-se que a exposição à fumaça da queima da cana-de-açúcar por diversos períodos de exposição em ratos Wistar esteve associada ao aumento do processo inflamatório no tecido traqueal e pulmonar. Observou-se a presença de infiltrados inflamatórios focais e difusos na fase aguda, com células polimorfonucleares na traqueia dos grupos expostos, principalmente no GE7. Não houve perda de epitélio ciliado em nenhum dos grupos experimentais, quando comparados com o GC. A fibrose tecidual na traqueia foi confirmada no GE21, que correspondeu aos estágios iniciais da fase crônica. No parênquima pulmonar, observaram-se alterações alveolares, vasculares e bronquiolares nos grupos expostos à fumaça, quando comparados com o GC. Os critérios temporais adotados para designar as fases da resposta inflamatória foram baseados em um estudo com ratos Wistar submetidos a sulfato de bleomicina, no qual se caracterizou a resposta inflamatória em aguda, subaguda e de resolução, nos períodos pós-lesão compreendidos entre o 1º e o 7º dia, do 7º ao 14º dia e a partir do 15º até o 30º dia, respectivamente.(17)
Estudos experimentais observaram inflamação pulmonar caracterizada por infiltrados de macrófagos e neutrófilos, após instilarem MP fino por via intratraqueal em ratos, demonstrando que as citocinas (IL-12 e IFN-γ) têm um papel fundamental na gravidade das lesões,(18) assim como, em coelhos, verificou-se um maior recrutamento de macrófagos e de células polimorfonucleares no parênquima pulmonar.(19) No presente estudo, verificaram-se alterações inflamatórias, constituídas de células polimorfonucleares e macrófagos alveolares no tecido pulmonar dos ratos a partir do GE1.
Pequenas doses de MP da queima da cana-de-açúcar instilados na traqueia geraram alterações no aparelho respiratório, com redução do espessamento do tecido conjuntivo e aumento da produção de citocinas e quimiocinas pró-inflamatórias.(8) No presente estudo, foi confirmado pela morfometria que a fumaça da queima da cana-de-açúcar é capaz de induzir processos necrótico-inflamatórios significantes e progressivos, mesmo em curtos períodos de exposição. Macrófagos alveolares após a fagocitose, assim como células epiteliais do pulmão, demonstram atividade responsiva à exposição de MP com amplificação na produção dos mediadores inflamatórios, podendo levar a certos mecanismos, tais como proliferação e ativação leucocitária, apoptose e reparo endotelial.(20) Essas alterações são justificadas devido aos elementos presentes na fumaça, dados que confirmam nossos achados, principalmente no GE7.
É sabido que o tamanho das partículas presentes na fumaça da queima da biomassa tem influência negativa sobre vias aéreas.(21,22) O tamanho do MP inalado determina as manifestações clínicas no organismo quanto à granularidade variável das partículas. O MP grosso (< 10 µm) fica retido na via área superior e pode ser removido pela atividade ciliar; os MPs finos (< 2,5 µm) e ultrafinos/nanopartículas (< 0,1 µm) são geralmente resultantes da oxidação incompleta de materiais de carbono.(22-24) Os MPs finos/ultrafinos possuem a capacidade de chegar aos alvéolos e ser submetidos à fagocitose pelos macrófagos alveolares, apresentando maiores efeitos deletérios, como alterações na mecânica pulmonar, colapso alveolar e estresse oxidativo, em ratos.(23-26) Exposições crônicas às partículas finas estão altamente associadas a maiores índices de doenças respiratórias crônicas.(3)
Na exposição direta da fumaça de biomassa (esterco) em camundongos durante 7 dias consecutivos, observou-se um aumento significativo da celularidade, principalmente de macrófagos, nos pulmões.(27) A exposição à fumaça da cana-de-açúcar gerou infiltrados inflamatórios de intensidades variadas nos animais do presente estudo, sendo que, no GE7, houve um infiltrado inflamatório mais significativo tanto em tecido traqueal como em parênquima pulmonar, pois houve áreas de inflamação com maior gravidade.
Os danos no parênquima pulmonar exposto cronicamente à fumaça de biomassa (esterco) com a fumaça de cigarro incluem inflamação intersticial de moderada a intensa, inflamação perivascular e brônquica, congestão vascular, destruição alveolar, aumento do número de macrófagos e aumento da espessura da parede vascular.(3) No presente estudo, apesar de a exposição ocorrer apenas na forma aguda, foi possível notar uma crescente alteração da estrutura alveolar (perdas septais) na comparação com o GC, sendo o MP fagocitado principalmente no GE1. Áreas sugestivas de necrose foram identificadas principalmente em GE7 e GE21, e a angiogênese foi confirmada no GE21.
Coelhos expostos cronicamente à queima de biomassa e de cigarro apresentam inflamação perivascular, peribronquiolar, infiltrado no parênquima e fibrose, com proliferação do epitélio respiratório e alterações enfisematosas.(28) Demonstramos, pela técnica de picrosírius, que, no GE7 e GE21, houve áreas de depósitos de colágeno no tecido traqueal, nas regiões peribronquiolar e vascular, assim como em áreas difusas do parênquima pulmonar, que sugerem que a exposição continuada pode levar ao processo fibrótico tecidual.
Em relação a alterações na estrutura alveolar no GE7 e a ruptura de septos interalveolares no GE21, constatamos por critérios morfológicos alterações condizentes com um estudo realizado em ratos que revelou um aumento dos espaços alveolares no grupo que foi exposto à fumaça do cigarro em 24,6% dos animais quando comparados com o GC, que não foi exposto.(29)
Portanto, com base nos dados obtidos, podemos afirmar que a exposição aguda à fumaça da queima da cana-de-açúcar é capaz de induzir graves danos ao sistema respiratório. Os componentes presentes na fumaça da cana-de-açúcar desencadeiam processos inflamatórios na traqueia com a presença de células polimorfonucleares e induzem também infiltrado inflamatório no parênquima pulmonar de ratos Wistar, com a presença de edemas intersticiais e alveolares. Alterações da arquitetura alveolar e angiogênese foram também evidenciadas.
Pesquisas adicionais tendo como alvo a exposição prolongada e a dosagem de marcadores pró-inflamatórios necessitam ser realizadas a fim de demonstrar os possíveis danos causados pela exposição crônica dos componentes presentes na fumaça da cana-de-açúcar, uma vez que os trabalhadores dos canaviais e a população circunvizinha ficam expostos a essa por longos períodos da vida.
REFERÊNCIAS1. Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Anuário Estatístico da Agroenergia 2014. Brasília: o Ministério; 2015.
2. Lelieveld J. Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367-71. https://doi.org/10.1038/nature15371
3. Dogan OT, Elagoz S, Ozsahin SL, Epozturk K, Tuncer E, Akkurt I. Pulmonary toxicity of chronic exposure to tobacco and biomass smoke in rats. Clinics (Sao Paulo). 2011;66(6):1081-7. https://doi.org/10.1590/S1807-59322011000600027
4. Ribeiro H. Sugar cane burning in Brazil: respiratory health effects [Article in Portuguese]. Rev Saude Publica. 2008;42(2):370-6. https://doi.org/10.1590/S0034-89102008005000009
5. Brasil. Ministério da Agricultura, Pecuária e Abastecimento [homepage on the Internet]. Brasília: o Ministério [cited 2016 Feb 10]. Sapcana - Sistema de Acompanhamento de Produção Canavieira. [Adobe Acrobat document, 3p.]. Available from: http://www.agricultura.gov.br/assuntos/sustentabilidade/agroenergia/acompanhamento-da-producao-sucroalcooleira/arquivos-2014-2015/16-04-2014.pdf/view
6. The WHO Pages. Wildland/Vegetation Fires. Ann Burns Fire Disasters [serial on the Internet]. 2000 [cited 2016 Feb 10] 13(3):178 [about 6 p.]. Available from: www.medbc.com/annals/review/vol_13/num_3/text/vol13n3p178.htm
7. Ribeiro H, Ficarelli TR. Queimadas nos canaviais e perspectivas dos cortadores de cana-de-açúcar em Macatuba, São Paulo. Saude Soc. 2010;9(1)48-63. http://dx.doi.org/10.1590/S0104-12902010000100005 https://doi.org/10.1590/S0104-12902010000100005
8. Bittar OT. Estudo dos efeitos do material particulado proveniente da biomassa de cana-de-açúcar sobre o aparelho respiratório e circulatório de ratos Wistar combinados ou não com a infecção estafilocócica [thesis]. Campinas: Universidade Estadual de Campinas; 2013.
9. Hutton G. The economics of health and climate change: key evidence for decision making. Global Health. 2011;7:18. https://doi.org/10.1186/1744-8603-7-18
10. National Aeronautics and Space Administration [homepage on the Internet]. Washington, DC: the Administration; c2015 [updated 2015 Jul 30; cited 2016 Feb 10]. A Story of Ozone: The Earth's natural sunscreen. Available from: https://www.nasa.gov/content/goddard/a-story-of-ozone-earths-natural-sunscreen
11. Prado GF, Zanetta DM, Arbex MA, Braga AL, Pereira LA, de Marchi MR, et al. Burnt sugarcane harvesting: Particulate matter exposure and the effects on lung function, oxidative stress, and urinary 1-hydroxypyrene. Sci Total Environ. 2012;437:200-8. https://doi.org/10.1016/j.scitotenv.2012.07.069
12. Arbex MA, Santos Ude P, Martins LC, Saldiva PH, Pereira LA, Braga AL. Air pollution and the respiratory system. J Bras Pneumol. 2012;38(5):643-55. https://doi.org/10.1590/S1806-37132012000500015
13. Mazzole-Rocha F, Carvalho GM, Lanzetti M, Valença SS, Silva LF, Saldiva PH et al. Respiratory toxicity of repeated exposure to particles produced by traffic and sugar cane burning. Respir Physiol Neurobiol. 2014;191:106-13. https://doi.org/10.1016/j.resp.2013.11.004
14. William AH, Wallace A. Simpson J, Hirani N. Acute lung injury. In: Hasleton P, Flieder DB, editors. Spencer's Pathology of the Lung. 6th ed. Cambridge: Cambridge University Press; 2013.p. 342-60.
15. Paraiso ML, Gouveia N. Health risks due to pre-harvesting sugarcane burning in São Paulo State, Brazil. Rev Bras Epidemiol. 2015;18(3):691-701. https://doi.org/10.1590/1980-5497201500030014
16. Silva R, Oyarzún M, Olloquequi J. Pathogenic mechanisms in chronic obstructive pulmonary disease due to biomass smoke exposure. Arch Bronconeumol. 2015;51(6)285-92. https://doi.org/10.1016/j.arbr.2015.04.013
17. Rossari JR. Fibrose pulmonar induzida por bleomicina intratraqueal em ratos Wistar: uso do interferon-α-2b em um modelo experimental de síndrome da distração respiratória aguda [dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2004.
18. Ding LR, Wang K, Fahmy B, Shen HH, Cormier S. Airborne fine particle matter induced pulmonary inflammation as well as oxidative stress in neonate rats. Chin Med J (Engl). 2010;123(20):2895-900.
19. Miyata R, Bai N, Vincent R, Sin DD, Van Eeden SF. Statins reduce ambient particulate matter-induced lung inflammation by promoting the clearance of particulate matter, < 10 µm from lung tissues. Chest. 2013;143(2):452-60. https://doi.org/10.1378/chest.12-1237
20. Poma A, Limongi T, Pisan C, Granato V, Picozzi P. Genotoxicity induced by fine urban air particulate matter in the macrophages cell line RAW 264.7. Toxicol In Vitro. 2006;20(6):1023-9. https://doi.org/10.1016/j.tiv.2006.01.014
21. Bovo F, Wisniewski P. Efeitos de material particulado sobre mecanismos imunológicos. Biosaude. 2009;11(2):93-100.
22. Donaldson K, Stone V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann Ist Super Sanita. 2003;39(3):405-10.
23. Pope CA, Muhlestein JB, Anderson JL, Cannon JB, Hales NM, Meredith KG, et al Short-Term Exposure to Fine Particulate Matter Air Pollution Is Preferentially Associated With the Risk of ST-Segment Elevation Acute Coronary Events. J Am Heart Assoc. 2015;4(12). pii: e002506. https://doi.org/10.1161/JAHA.115.002506
24. Colombini MP. Exposição aguda ao material particulado total em suspensão proveniente de diferentes fontes e suas repercussões nas respostas inflamatórias, sistêmica e local, em ratos [thesis]. São Paulo. Faculdade de Medicina, Universidade de São Paulo; 2007.
25. Mazzoli-Rocha F, Magalhães CB, Malm O, Saldiva PH, Zin WA, Faffe DS. Comparative respiratory toxicity of particles produced by traffic and sugar cane burning. Environ Res. 2008;108(1):35-41. https://doi.org/10.1016/j.envres.2008.05.004
26. Riva DR, Magalhães CB, Lopes AA, Lanças T, Mauad T, Malm O, et al. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol. 2011;23(5):257-67. https://doi.org/10.3109/08958378.2011.566290
27. Mehra D, Geraghty PM, Hardigan AA, Foronjy R. A comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PLoS One. 2012;7(12):e52889. https://doi.org/10.1371/journal.pone.0052889
28. Fidan F, Unlu M, Sezer M, Sahin O, Tokyol C, Esme H. Acute effects of environmental tobacco smoke and dried dung smoke on lung histopathology in rabbits. Pathology. 2006;38(1):53-7. https://doi.org/10.1080/00313020500459615
29. Zheng H, Liu Y, Huang T, Fang Z, Li G, He S. Development and characterization of a rat model of chronic obstructive pulmonary disease (COPD) in-duced by sidestream cigarette smoke. Toxicol Lett. 2009;189(3):225-34. https://doi.org/10.1016/j.toxlet.2009.06.850








