ABSTRACT
Objective: To describe diagnostic and treatment aspects of hard metal lung disease (HMLD) and to review the current literature on the topic. Methods: This was a retrospective study based on the medical records of patients treated at the Occupational Respiratory Diseases Clinic of the Instituto do Coração, in the city of São Paulo, Brazil, between 2010 and 2013. Results: Of 320 patients treated during the study period, 5 (1.56%) were diagnosed with HMLD. All of those 5 patients were male (mean age, 42.0 ± 13.6 years; mean duration of exposure to hard metals, 11.4 ± 8.0 years). Occupational histories were taken, after which the patients underwent clinical evaluation, chest HRCT, pulmonary function tests, bronchoscopy, BAL, and lung biopsy. Restrictive lung disease was found in all subjects. The most common chest HRCT finding was ground glass opacities (in 80%). In 4 patients, BALF revealed multinucleated giant cells. In 3 patients, lung biopsy revealed giant cell interstitial pneumonia. One patient was diagnosed with desquamative interstitial pneumonia associated with cellular bronchiolitis, and another was diagnosed with a hypersensitivity pneumonitis pattern. All patients were withdrawn from exposure and treated with corticosteroid. Clinical improvement occurred in 2 patients, whereas the disease progressed in 3. Conclusions: Although HMLD is a rare entity, it should always be included in the differential diagnosis of respiratory dysfunction in workers with a high occupational risk of exposure to hard metal particles. A relevant history (clinical and occupational) accompanied by chest HRCT and BAL findings suggestive of the disease might be sufficient for the diagnosis.
Keywords:
Lung diseases, interstitial; Cobalt; Tungsten; Occupational exposure; Hard metal.
RESUMO
Objetivo: Descrever aspectos relacionados ao diagnóstico e tratamento de pacientes com doença pulmonar por metal duro (DPMD) e realizar uma revisão da literatura. Métodos: Estudo retrospectivo dos prontuários médicos de pacientes atendidos no Serviço de Doenças Respiratórias Ocupacionais do Instituto do Coração, localizado na cidade de São Paulo, entre 2010 e 2013. Resultados: Entre 320 pacientes atendidos no período do estudo, 5 (1,56%) foram diagnosticados com DPMD. Todos os pacientes eram do sexo masculino, com média de idade de 42,0 ± 13,6 anos e média de tempo de exposição a metal duro de 11,4 ± 8,0 anos. Os pacientes foram submetidos a avaliação clinica, história ocupacional, TCAR de tórax, prova de função pulmonar, broncoscopia com LBA e biópsia pulmonar. Todos apresentaram distúrbio ventilatório restritivo. O achado de imagem à TCAR de tórax mais frequente foi de opacidades em vidro fosco (em 80%). Em 4 pacientes, o LBA revelou presença de células gigantes multinucleadas. Em 3, foi diagnosticada pneumonia intersticial por células gigantes na biópsia pulmonar. Houve o diagnóstico de pneumonia intersticial descamativa associada à bronquiolite celular em 1 paciente e de pneumonite de hipersensibilidade em 1. Todos foram afastados da exposição e tratados com corticoide. Houve melhora em 2 pacientes e progressão da doença em 3. Conclusões: Apesar de ser uma entidade rara, a DPMD deve ser sempre considerada em trabalhadores com risco ocupacional elevado de exposição a metais duros. A história clínica e ocupacional associada a achados em TCAR de tórax e LBA sugestivos da doença podem ser suficientes para o diagnóstico.
Palavras-chave:
Doenças pulmonares intersticiais; Cobalto; Tungstênio; Exposição ocupacional; Metal duro.
INTRODUCTIONHard metal lung disease (HMLD) is a rare disease caused by exposure to particles of hard metal alloys, whose ma-jor components are tungsten carbide (approximately 90%) and cobalt (approximately 10%) or cobalt and dia-mond. (1,2) Other components , such as tantalum, titanium, nickel, niobium, and chrome, are found in small amounts.
Because they are extremely hard and maintain their physical properties even at high temperatures, hard metals are used in tools for cutting and sharpening metals, drilling wells, polishing diamonds and dental prostheses, etc. Workers are exposed to particles rich in cobalt (in ionized form) and tungsten carbide, which are absorbed by the lungs and gastrointestinal tract, both in cobalt powder production and when using cobalt alloy tools. The pathophysi-ology of HMLD remains unknown. The proposed mechanism for the pathogenesis of the interstitial disease involves a hypersensitivity reaction to cobalt.(3) In addition, genetic susceptibility(4) may play a role in the pathogenesis of the disease, although this role has yet to be fully understood.
Exposure to cobalt can cause several forms of lung disease, from asthma to various interstitial patterns in the lungs.(1) The most widely known and typical histopathological presentation is giant cell interstitial pneumonia (GIP), described by Liebow(5) in 1968. GIP is characterized by the presence of multinucleated giant cells, which can be "macrophagic" in nature,(1,6) in alveolar spaces. In addition to GIP, other patterns described include usual interstitial pneumonia, hypersensitivity pneumonitis (HP), and desquamative interstitial pneumonia.(1,6) In the present study, we describe diagnostic and treatment aspects of HMLD and review the current literature on the topic.
METHODSThe study subjects were treated at the Occupational Respiratory Diseases Clinic of the Pulmonology Division of the Instituto do Coração of the University of São Paulo School of Medicine Hospital das Clínicas, in the city of São Paulo, Brazil, between 2010 and 2013. Occupational histories were taken, after which all subjects underwent clinical evalua-tion, chest HRCT, pulmonary function tests (Elite DX Series, Medical Graphics Corporation, Saint Paul, MN, USA), bronchoscopy, BAL, and lung biopsy. When the lung tissue obtained was considered insufficient for diagnosis, surgi-cal biopsies were performed. We performed elemental analysis of lyophilized lung tissue specimens by energy-dispersive X-ray fluorescence spectrometry(7) in 2 patients (EDX 700-HS, Shimadzu Corporation, Analytical Instru-ments Division, Kyoto, Japan).
RESULTSDuring the study period, 320 patients were treated at the facility. Of those, 5 (1.56%) were diagnosed with HMLD. The mean age at diagnosis was 42.0 ± 13.6 years. All of those 5 patients were male and were working at the time of the initial evaluation. The mean duration of exposure was 11.4 ± 8.0 years. One patient reported working as an industrial tool maintenance technician, 2 reported being industrial tool sharpeners, and 2 reported being grinder operators (Table 1). All 5 clinically presented with dyspnea on exertion and cough.
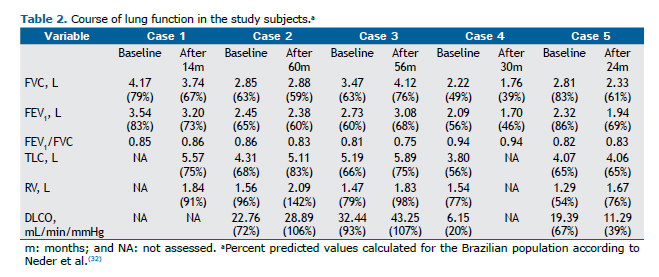
The most common chest HRCT finding was ground glass opacities, in 4 patients (80%; Figure 1). Other findings in-cluded peripheral reticular opacities with traction bronchiectasis and bronchiolectasis, in 3 (60%; Figure 2); mi-cronodules, in 2 (40%; Figure 2); and bronchial wall thickening, in 2 (40%; Table 1). Restrictive lung disease, in varying degrees, was found in all subjects, and decreased DLCO was found in 3 (Table 2).



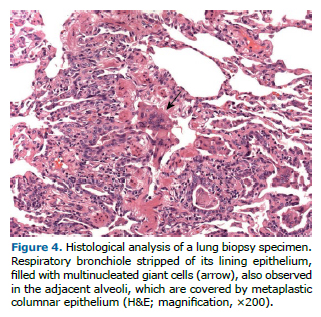
All patients underwent bronchoscopy, BAL, and lung biopsy, with the diagnosis being established by examination of a bronchoscopy specimen in 1 patient and by examination of a surgical biopsy specimen in 4. Large numbers of multinucleated giant cells were found in the BAL fluid of 4 of the 5 patients (Figure 3) and in the biopsy tissue of 3 (Figure 4). Desquamative interstitial pneumonia associated with cellular bronchiolitis was diagnosed in 1 patient; a histological pattern suggestive of chronic HP, with no evidence of other occupational or environmental exposures that could be associated with HP, was identified in 1; the presence of tungsten was identified in 2, whose tissue samples were analyzed by energy-dispersive X-ray fluorescence spectrometry; and the presence of cobalt was identified in 1 of those 2 (Table 1).

Following diagnosis, all patients were withdrawn from their occupational exposure and received systemic cortico-steroid therapy. Two patients showed clinical, radiological, and functional improvement, although their lung function did not normalize (Tables 1 and 2). Three patients experienced disease progression despite corticosteroid therapy, 2 of whom additionally received azathioprine. One patient was placed on a lung transplant list, but he died before undergoing transplantation.
DISCUSSIONIn the case series presented here, ground-glass opacities were the most common chest HRCT changes. Restrictive lung disease was the most common finding, occurring in all patients. Multinucleated giant cells were detected in the BAL fluid of most patients, and this finding was correlated with the typical histological pattern of GIP. Withdrawal from exposure combined with corticosteroid therapy was successful in 2 patients, and disease progression occurred in 3 patients.
The functional, cytological, histopathological, and imaging findings are similar to those found in other studies.(1,6,8)
HMLD is a rare entity, even in populations at occupational risk. The published literature on cases of HMLD in Brazil is scarce and consists of the reporting of the disease in 4 patients.(9-12) In the literature, data on the prevalence and incidence of HMLD among individuals exposed to hard metals vary. Meyer-Bisch et al.(13) and Kusaka et al.(14) found no cases of HMLD in cross-sectional studies involving 425 and 319 workers, respectively, with a mean duration of exposure to hard metals of 9 and 14 years, respectively. Sprince et al.(15) found only 1 patient with HMLD among 290 hard metal production workers evaluated. The diagnostic imaging methods available at the time might have contrib-uted to the low HMLD detection results observed in those studies.(13-15)
The paucity of cases and the varying latency period suggest that a hypersensitivity mechanism may be involved in the pathophysiology of HMLD. Sabbioni et al.(3) found that 9% of the workers who had contact with hard metals had lung disease, and that the observed prevalence was not correlated with cobalt, tungsten, or tantalum levels, nor was it correlated with age, gender, or duration of occupational exposure, which, in the authors' view, reinforced the hy-persensitivity hypothesis. Recurrence of HMLD in unilateral(16,17) or bilateral(6) lung transplant recipients, even after cessation of exposure to hard metals, suggests the hypothesis that immune-mediated mechanisms are involved in the pathophysiology of the disease. In those 3 reported cases of recurrence,(6,16,17) the histological pattern of GIP was found, and no traces of tungsten or cobalt were detected in the transplanted lungs, which, in the authors' view, rein-forced this immune-mediated hypothesis.
Genetic susceptibility also contributes to the development of HMLD. Potolicchio et al.(4) demonstrated that HLA-DP-βGlu69 was more prevalent among hard-metal-exposed workers with HMLD than in those without. In that study,(4) 19 (95%) of 20 hard-metal-exposed workers with HMLD exhibited this polymorphism, compared with 17 (48.6%) of 35 hard-metal-exposed workers without the disease.
The individual contribution of the major components of hard metal has also been investigated. In animal models, instillation of tungsten alone did not produce pulmonary inflammation, whereas cobalt alone was able to produce tissue injury. When combined, cobalt and tungsten had a synergistic effect and caused increased tissue inflamma-tion.(18,19)
Moriyama et al.(8) performed electron microprobe analysis of elemental levels in 17 patients with HMLD and con-cluded that cobalt and tungsten accumulate in centrilobular fibrotic lesions. In that study, immunohistochemistry revealed the presence of tungsten particles within lesions surrounded by CD163+ macrophages and T CD8+ lym-phocytes. The authors(8) suggest that such macrophages play an important role in the development of fibrotic lesions.
The clinical presentation of HMLD varies widely, as shown in this case series. The initial symptom is usually dysp-nea on exertion. Patients who have HMLD develop restrictive lung disease and have a reduction in DLCO, of varying severity.(1)
A relevant clinical and occupational history is essential for the diagnosis of HMLD. Although chest X-ray and HRCT findings are nonspecific, they are important in diagnosis, together with a history of exposure and histopathological changes. The major chest HRCT changes found by Choi et al.(20) were ground-glass opacities and irregular linear opacities, predominantly in the lower lung fields; these changes were also commonly seen in the 5 cases described here.
Unlike in other pneumoconioses, in which, in most cases, relevant occupational history and suggestive chest X-ray findings are sufficient to establish the diagnosis, in HMLD, chest HRCT and a specimen for cytological or histological analysis are required. BAL, which is a minimally invasive method, can confirm the diagnosis without the need for lung biopsy.(1,21)
Forni,(21) in reviewing BAL cytopathological findings described in the literature, concluded that there are two pat-terns that suggest the diagnosis of HMLD. The first is characterized by significantly increased cellularity, resulting from characteristic multinucleated giant cells, lymphocytosis with a decreased CD4/CD8 ratio, and eosinophilia. To Forni,(21) these findings mirror the histopathological findings of GIP, and, in such cases, a lung tissue biopsy would not be required. The second BAL pattern is that of predominant lymphomononuclear infiltrates, with a decreased CD4/CD8 ratio, rare eosinophils, and giant cells, being similar to the pattern found in HP. In such cases, biopsy is essential to establish the diagnosis.(21)
In the present case series, the BAL patterns coincided with those described by Forni(21) and reinforce the relation-ship between the presence of multinucleated giant cells in the BAL and the histological diagnosis of GIP.
Naqvi et al.,(1) in a review of 100 patients who had undergone lung biopsy and had a diagnosis of HMLD, reported that 59 of those patients had the pattern of GIP, defined as the presence of typical multinucleated giant cells in the alveolar space. Moriyama et al.(8) highlighted the importance of the presence of centrilobular fibrotic lesions in the histological specimen for the diagnosis of HMLD, even when there are no multinucleated giant cells. Another histo-pathological pattern that is seen is that of HP.(1,22) Hard-metal-exposed workers often also use cutting oil, which may be contaminated with Mycobacterium immunogenum, whose antigens can cause HP.(23)
When the observed histological patterns are inconsistent with GIP, elemental analysis, by a combination of electron microscopy and energy-dispersive X-ray fluorescence spectrometry, is important in establishing the diagnosis.(1) Tungsten is usually found at high levels,(1,8,24) whereas cobalt is usually found at moderate or low levels, since cobalt is highly soluble and is eliminated rapidly.(1,3)
Although some authors have suggested that GIP is a pathognomonic finding of HMLD,(25) there have been several published reports of cases(1,6,8) with a histological pattern of GIP without occupational exposure to hard metals and without tungsten or cobalt being detected in lung tissue. Other authors(8,26) have suggested that such cases corre-spond to idiopathic GIP. Other reported cases of GIP have been associated with exposure to titanium(27) and to nitro-furantoin.(28)
Treatment of HMLD consists of withdrawal from the occupational exposure, which can stabilize or even improve lung function,(29) and corticosteroid therapy. Because of the scarcity of cases in the literature, there have been no studies comparing corticosteroid therapy and placebo treatment; however, clinical experience is that corticosteroid therapy results in improvement.(9,30) The use of immunosuppressive therapy also is not well established, although immunosuppressants are occasionally used.(9) Lung transplantation is a treatment option in patients with advanced lung disease that progresses despite treatment,(31) despite the possibility of GIP pattern recurrence in the transplant-ed lung.(6,16,17)
Although HMLD is rare, it should always be included in the differential diagnosis of respiratory dysfunction in hard-metal-exposed workers-such as tool sharpeners, toolmakers, operators of machines (such as milling cutters, grind-ers, and lathes), operators of diamond-blade cutting discs, and diamond polishers (using polishing discs made of hard metals)-who present with clinical complaints, functional changes, or imaging changes. Data in the literature and our experience with the cases reported here suggest that adequate occupational history taking accompanied by chest HRCT changes and BAL findings (lymphocytosis with a decreased CD4/CD8 ratio and, especially, the presence of large numbers of giant cells) consistent with the disease might be sufficient for the diagnosis, there being no need for an open lung biopsy. Treatment is based on permanent withdrawal from exposure and corticosteroid or immunosup-pressive therapy, with varying results.
REFERENCES1. Naqvi AH, Hunt A, Burnett BR, Abraham JL. Pathologic spectrum and lung dust burden in giant cell interstitial pneumonia (hard metal disease/cobalt pneumonitis): review of 100 cases. Arch Environ Occup Health. 2008;63(2):51-70. https:/doi.org/10.3200/AEOH.63.2.51-70
2. Palmen N. Criteria Document for Swedish Occupational Standards: Cobalt and Cobalt Compounds. Stockholm: Swedish National Institute for Working Life, Swedish Criteria Group for Occupational Standards; 2015. Report No.: NR 2005:12.
3. Sabbioni E, Minoia C, Pietra R, Mosconi G, Forni A, Scansetti G. Metal determinations in biological specimens of diseased and non-diseased hard metal workers. Sci Total Environ. 1994;150(1-3):41-54. https:/doi.org/10.1016/0048-9697(94)90127-9
4. Potolicchio I, Mosconi G, Forni A, Nemery B, Seghizzi P, Sorrentino R. Susceptibility to hard metal lung disease is strongly associated with the presence of glutamate 69 in HLA-DP beta chain. Eur J Immunol. 1997;27(10):2741-3. https:/doi.org/10.1002/eji.1830271039
5. Liebow A. New Concepts and Entities in Pulmonary Disease in the Lung. Baltimore, MD: Williams and Wilkins; 1968. p. 332.
6. Khoor A, Roden AC, Colby TV, Roggli VL, Elrefaei M, Alvarez F et al. Giant cell interstitial pneumonia in patients without hard metal exposure: analysis of 3 cases and review of the literature. Hum Pathol. 2016;50:176-82. https:/doi.org/10.1016/j.humpath.2015.12.004
7. Carneiro MF, Ribeiro FQ, Fernandes-Filho FN, Lobo DJ, Barbosa F Jr, Rhoden CR, et al. Pollen abortion rates, nitrogen dioxide by passive diffusive tubes and bioaccumulation in tree barks are effective in the characterization of air pollution. Environ Exp Bot. 2011;72(2):272-7. https:/doi.org/10.1016/j.envexpbot.2011.04.001
8. Moriyama H, Kobayashi M, Takada T, Shimizu T, Terada M, Narita J, et al. Two-dimensional analysis of elements and mononuclear cells in hard metal lung disease. Am J Respir Crit Care Med. 2007;176(1):70-7. https:/doi.org/10.1164/rccm.200601-134OC
9. Bezerra PN, Vasconcelos AG, Cavalcante LL, Marques VB, Nogueira TN, Holanda MA. Hard metal lung disease in an oil industry worker. J Bras Pneumol. 2009;35(12):1254-8.
10. Moreira MA, Cardoso Ada R, Silva DG, Queiroz MC, Oliveira AA, Noleto TM. Hard metal pneumoconiosis with spontaneous bilateral pneumothorax. J Bras Pneumol. 2010;36(1):148-151. https:/doi.org/10.1590/S1806-37132010000100020
11. De Capitani EM, Schweller M, Grangeial TA, Corso-Pereira M, Cerqueira EM, Metze K, et al. Pneumonia intersticial descamativa secundária a exposição à poeira de metal duro. Pneumologia Paulista, 2010;24(11):62-3.
12. De Capitani EM, Corso-Pereira M, Saldiva PH. Hard metal subacute pneumopathy: a case report. In: Proceedings of the 8th International Conference on Occupational Lung Diseases; 1992 Sep 14-17; Prague. ILO and Czech Medical Society; 1993.
13. Meyer-Bisch C, Pham QT, Mur JM, Massin N, Moulin JJ, Teculescu D, et al. Respiratory hazards in hard metal workers: a cross sectional study. Br J Ind Med. 1989;46(5):302-9. https:/doi.org/10.1136/oem.46.5.302
14. Kusaka Y, Yokoyama K, Sera Y, Yamamoto S, Sone S, Kyono H, et al. Respiratory diseases in hard metal workers: an occupational hygiene study in a factory. Br J Ind Med. 1986;43(7):474-85. https:/doi.org/10.1136/oem.43.7.474
15. Sprince NL, Chamberlin RI, Hales CA, Weber AL, Kazemi H. Respiratory disease in tungsten carbide production workers. Chest. 1984;86(4):549-57. https:/doi.org/10.1378/chest.86.4.549
16. Frost AE, Keller CA, Brown RW, Noon GP, Short HD, Abraham JL, et al. Giant cell interstitial pneumonitis. Disease recurrence in the transplanted lung. Am Rev Respir Dis. 1993;148(5):1401-4. https:/doi.org/10.1164/ajrccm/148.5.1401
17. Tarabichi Y, Saggar R, Wallace WD, Lynch JP 3rd, Saggar R. Primary disease recurrence after single lung transplantation in a patient with prior hard metal exposure. J Heart Lung Transplant. 2015;34(9):1216-8. https:/doi.org/10.1016/j.healun.2015.05.019
18. Kaplun ZS, Mezencheva NV. Experimental study on the toxic effect of dust in production of sintered metals [Article in German]. J Hyg Epidemiol Microbiol Immunol. 1960;4:390-9.
19. Lasfargues G, Lardot C, Delos M, Lauwerys R, Lison D. The delayed lung responses to single and repeated intratracheal administration of pure cobalt and hard metal powder in the rat. Environ Res. 1995;69(2):108-21. https:/doi.org/10.1006/enrs.1995.1032
20. Choi JW, Lee KS, Chung MP, Han J, Chung MJ, Park JS. Giant cell interstitial pneumonia: high-resolution CT and pathologic findings in four adult patients. Am J Roentgenol. 2005;184(1):268-72. https:/doi.org/10.2214/ajr.184.1.01840268
21. Forni A. Bronchoalveolar lavage in the diagnosis of hard metal disease. Sci Total Environ. 1994;150(1-3):69-76. https:/doi.org/10.1016/0048-9697(94)90131-7
22. Okuno K, Kobayashi K, Kotani Y, Ohnishi H, Ohbayashi C, Nishimura Y. A case of hard metal lung disease resembling a hypersensitive pneumonia in radiological images. Inter Med. 2010;49(12):1185-9. https:/doi.org/10.2169/internalmedicine.49.3049
23. Tillie-Leblond I, Grenouillet F, Reboux G, Roussel S, Chouraki B, Lorthois C, et al. Hypersensitivity pneumonitis and metalworking fluids contaminated by mycobacteria. Eur Respir J. 2011;37(3):640-7. https:/doi.org/10.1183/09031936.00195009
24. Tanaka J, Moriyama H, Terada M, Takada T, Suzuki E, Narita I, et al. An observational study of giant cell interstitial pneumonia and lung fibrosis in hard metal lung disease. BMJ Open. 2014;4(3):e004407. https:/doi.org/10.1136/bmjopen-2013-004407
25. Abraham JL. Lung pathology in 22 cases of giant cell interstitial pneumonia (GIP) suggests GIP is pathognomonic of cobalt (hard metal) disease [abstract]. Chest. 1987;91:312.
26. Blanc PD. Is giant cell interstitial pneumonitis synonymous with hard metal lung disease? Am J Respir Crit Care Med. 2007;176(8): 834; author reply 834-5. https:/doi.org/10.1164/ajrccm.176.8.834
27. Paris C, Pairon JC, Billon-Galland MA, Vanoni-Espiand H, Godbert B, Martinet Y, et al. Giant cell interstitial pneumonia: report of two cases with high titanium concentration in the lung. Am J Respir Crit Care Med. 2011;184(11):1315-7. https:/doi.org/10.1164/ajrccm.184.11.1315a
28. Lee B, Balavenkataraman A, Sanghavi D, Walter K. Recurrent nitrofurantoin-induced giant cell interstitial pneumonia: case report and literature review. Respir Med Case Rep. 2015;14:49-52. https:/doi.org/10.1016/j.rmcr.2015.01.002
29. Terui H, Konno S, Kaga K, Matsuno Y, Hatanaka KC, Kanno H, et al. Two cases of hard metal lung disease showing gradual improvement in pulmonary function after avoiding dust exposure. J Occup Med Toxicol. 2015;10:29. https:/doi.org/10.1186/s12995-015-0070-9
30. Enriquez LS, Mohammed TL, Johnson GL, Lefor MJ, Beasley MB. Hard metal pneumoconiosis: a case of giant-cell interstitial pneumonitis in a machinist. Respir Care. 2007;52(2):196-9.
31. Weill D, Bender C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1-15. https:/doi.org/10.1016/j.healun.2014.06.014
32. Neder JA, Andreoni S, Castelo-Filho A, Nery LE. Reference values for lung function tests. I. Static volumes. Braz J Med Biol Res. 1999;32(6):703-17. https:/doi.org/10.1590/s0100-879x1999000600006







