Luiz Fernando Ferreira Pereira1, Eliane Viana Mancuzo2, Camila Farnese Rezende3, Ricardo de Amorim Côrrea4
ABSTRACT
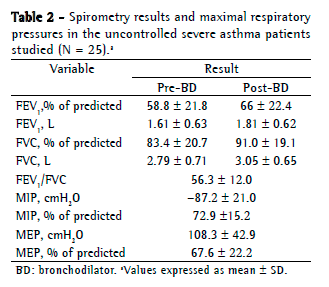
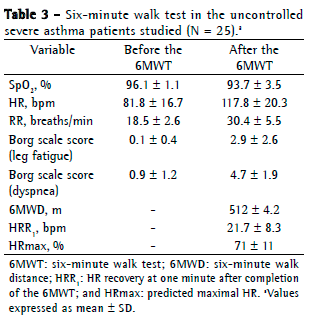
Objective: To evaluate respiratory muscle strength and six-minute walk test (6MWT) variables in patients with uncontrolled severe asthma (UCSA). Methods: This was a cross-sectional study involving UCSA patients followed at a university hospital. The patients underwent 6MWT, spirometry, and measurements of respiratory muscle strength, as well as completing the Asthma Control Test (ACT). The Mann-Whitney test was used in order to analyze 6MWT variables, whereas the Kruskal-Wallis test was used to determine whether there was an association between the use of oral corticosteroids and respiratory muscle strength. Results: We included 25 patients. Mean FEV1 was 58.8 21.8% of predicted, and mean ACT score was 14.0 3.9 points. No significant difference was found between the median six-minute walk distance recorded for the UCSA patients and that predicted for healthy Brazilians (512 m and 534 m, respectively; p = 0.14). During the 6MWT, there was no significant drop in SpO2. Mean MIP and MEP were normal (72.9 15.2% and 67.6 22.2%, respectively). Comparing the patients treated with at least four courses of oral corticosteroids per year and those treated with three or fewer, we found no significant differences in MIP (p = 0.15) or MEP (p = 0.45). Conclusions: Our findings suggest that UCSA patients are similar to normal subjects in terms of 6MWT variables and respiratory muscle strength. The use of oral corticosteroids has no apparent impact on respiratory muscle strength.
RESUMO
Objetivo: Avaliar a força muscular respiratória e variáveis obtidas no teste de caminhada de seis minutos (TC6) em pacientes com asma grave não controlada (AGNC). Métodos: Estudo transversal, envolvendo pacientes com AGNC acompanhados em um hospital universitário. Os pacientes foram submetidos a TC6, espirometria e medidas da força muscular respiratória e responderam o Asthma Control Test (ACT, Teste de Controle da Asma). O teste de Mann-Whitney foi utilizado na análise das variáveis do TC6, e o teste de Kruskal-Wallis foi utilizado na verificação de uma possível associação do uso de corticoide oral com a força muscular respiratória. Resultados: Foram incluídos 25 pacientes, com médias de VEF1 de 58,8 21,8% do previsto e escore do ACT de 14,0 3,9 pontos. Não houve diferença significativa entre a mediana da distância percorrida no TC6 dos pacientes com AGNC e aquela prevista para brasileiros saudáveis (512 m e 534 m, respectivamente; p = 0,14). Durante o TC6, não houve queda significativa da SpO2. As médias de PImáx e PEmáx foram normais (72,9 15,2% e 67,6 22,2%, respectivamente). Não houve diferenças significativas nas medidas de PImáx (p = 0,15) e PEmáx (p = 0,45) entre os pacientes que usavam ao menos quatro ciclos de corticoide oral por ano e os que o usavam por três ou menos ciclos por ano. Conclusões: Nossos achados sugerem que os pacientes com AGNC são semelhantes a indivíduos normais em termos das variáveis do TC6 e da força muscular respiratória. Não se observou um impacto do uso de corticoide oral na força muscular respiratória.
Palavras-chave: Asma; Tolerância ao exercício; Músculos respiratórios.
Introduction