Silvana Neves Ferraz de Assunção, Carla Hilário da Cunha Daltro, Ney Christian Boa Sorte, Hugo da Costa Ribeiro Júnior, Maria de Lourdes Bastos, Cleriston Farias Queiroz, Antônio Carlos Moreira Lemos
ABSTRACT
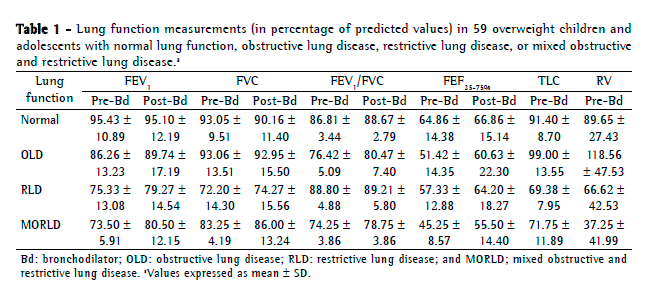
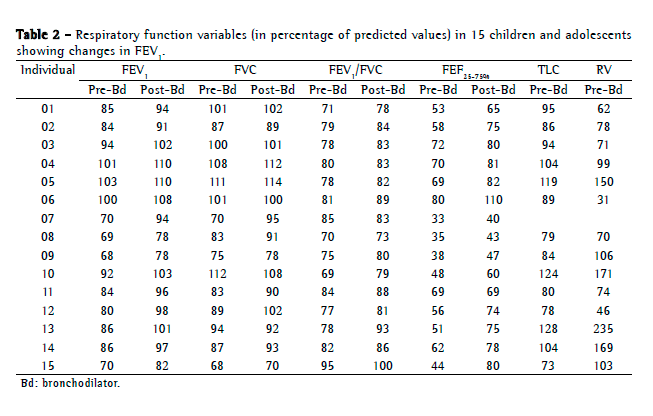
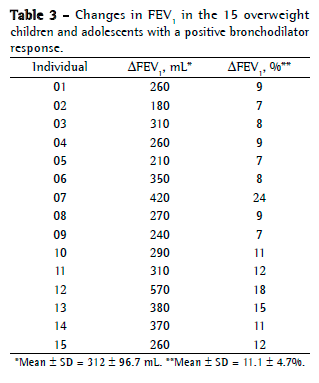
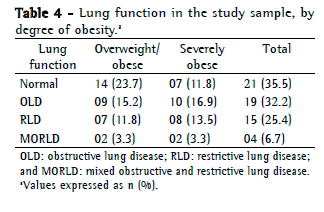
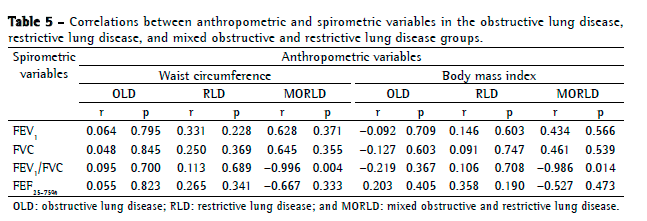
Objective: To describe lung function findings in overweight children and adolescents without respiratory disease. Methods: This was a cross-sectional study involving male and female overweight children and adolescents in the 8-18 year age bracket, without respiratory disease. All of the participants underwent anthropometric assessment, chest X-ray, pulse oximetry, spirometry, and lung volume measurements. Individuals with respiratory disease were excluded, as were those who were smokers, those with abnormal chest X-rays, and those with an SpO2 ≤ 92%. Waist circumference was measured in centimeters. The body mass index-for-age Z score for boys and girls was used in order to classify the individuals as overweight, obese, or severely obese. Lung function variables were expressed in percentage of the predicted value and were correlated with the anthropometric indices. Results: We included 59 individuals (30 males and 29 females). The mean age was 11.7 ± 2.7 years. Lung function was normal in 21 individuals (35.6%). Of the 38 remaining individuals, 19 (32.2%), 15 (25.4%), and 4 (6.7%) presented with obstructive, restrictive, and mixed ventilatory disorder, respectively. The bronchodilator response was positive in 15 individuals (25.4%), and TLC measurements revealed that all of the individuals with reduced VC had restrictive ventilatory disorder. There were significant negative correlations between the anthropometric indices and the Tiffeneau index in the individuals with mixed ventilatory disorder. Conclusions: Lung function was abnormal in approximately 65% of the individuals evaluated here, all of whom were overweight. Obstructive ventilatory disorder and positive bronchodilator response predominated.
Keywords: Obesity/complications; Respiratory function tests; Lung diseases/etiology.
RESUMO
Objetivo: Descrever os achados de função pulmonar em crianças e adolescentes sem doenças respiratórias e com excesso de peso. Métodos: Estudo transversal com crianças e adolescentes de 8 a 18 anos de ambos os sexos, com excesso de peso e sem doença respiratória, submetidos à avaliação antropométrica, radiografia de tórax, oximetria de pulso, espirometria e medidas de volume pulmonar. Indivíduos com patologias respiratórias, tabagistas ativos, radiografia anormal ou SpO2 ≤ 92% foram excluídos do estudo. A circunferência da cintura foi medida em centímetros. O escore z para índice de massa corpórea/idade e sexo foi utilizado para classificar os indivíduos como com sobrepeso, obesos e obesos graves. As variáveis dos testes de função pulmonar foram expressas em percentual do previsto e correlacionadas com os índices antropométricos. Resultados: Foram incluídos 59 indivíduos (30 meninos e 29 meninas). A média de idade foi de 11,7 ± 2,7 anos. Os resultados dos testes de função pulmonar foram normais em 21 indivíduos (35,6%). Dos 38 indivíduos restantes, 19 (32,2%), 15 (25,4%) e 4 (6,7%) apresentaram, respectivamente, distúrbio ventilatório obstrutivo, restritivo e misto. A resposta ao broncodilatador foi positiva em 15 indivíduos (25,4%), e a medida da CPT revelou que todos os indivíduos com CV reduzida apresentavam distúrbio ventilatório restritivo. Houve correlações negativas significantes entre os índices antropométricos e índice de Tiffeneau nos indivíduos com distúrbio ventilatório misto. Conclusões: A função pulmonar apresentou-se alterada em aproximadamente 65% dos indivíduos com sobrepeso aqui avaliados, predominando distúrbio ventilatório obstrutivo e resposta positiva ao broncodilatador.
Palavras-chave: Obesidade/complicações; Testes de função respiratória; Pneumopatias/etiologia.
Introduction