ABSTRACT
Objective: In individuals with asthma, obesity induces the production of leptin and is associated with disease severity. Our objective was to evaluate the levels of serum leptin and their effect on Th1/Th2 balance in obese and non-obese children with asthma, as well as to investigate the association between serum leptin levels and clinical outcomes. Methods: We evaluated 50 atopic children with physician-diagnosed moderate-to-severe persistent asthma and 20 controls. The children with asthma were divided into two groups, by body mass index percentile: obese (n = 25) and non-obese (n = 25). From all subjects, we collected peripheral blood samples in order to determine the levels of leptin, IFN-, and IL-4. Asthma severity was assessed by an asthma symptom score, and the results were correlated with the parameters studied. Results: Serum leptin levels were significantly higher in the obese asthma group than in the non-obese asthma group, as well as being significantly higher in the children with asthma than in the controls, whereas IFN- levels were significantly higher and IL-4 levels were significantly lower in the obese asthma group than in the non-obese asthma group. In addition, the obese asthma group showed higher asthma symptom scores and significantly lower FEV1 (% of predicted) than did the non-obese asthma group. There was a significant positive correlation between leptin and IFN- levels only in the obese asthma group. Conclusions: Although leptin is involved in the pathogenesis of asthma in obese and non-obese children, its effect is more pronounced in the former. In the presence of high leptin levels, only obese children with asthma exhibited Th1 polarization, with higher IFN- levels and greater asthma severity.
RESUMO
Objetivo: A obesidade induz a produção de leptina em asmáticos e está associada à gravidade da doença. Nosso objetivo foi avaliar os níveis de leptina sérica e seu efeito no equilíbrio Th1/Th2 em crianças asmáticas obesas e não obesas e investigar a associação desses níveis com desfechos clínicos. Métodos: O estudo envolveu 50 crianças atópicas com diagnóstico médico de asma persistente moderada a grave e 20 controles. Os asmáticos foram agrupados como obesos (n = 25) e não obesos (n = 25) de acordo com o percentil do índice de massa corpórea. Amostras de sangue periférico foram coletadas de todos os sujeitos, e os níveis de leptina, IFN- e IL-4 foram determinados. A gravidade da asma foi avaliada por um escore de sintomas de asma, e os resultados foram correlacionados com os parâmetros estudados. Resultados: Os níveis séricos de leptina foram significativamente maiores nos asmáticos obesos do que nos asmáticos não obesos, assim como nos asmáticos comparados aos controles, enquanto os níveis de IFN- foram significativamente maiores e os de IL-4 foram significativamente menores nos asmáticos obesos do que nos asmáticos não obesos. Os asmáticos obesos tiveram maiores escores de sintomas de asma e VEF1 (% do previsto) significativamente menor que os asmáticos não obesos. Houve uma correlação positiva significativa entre os níveis de leptina e IFN- somente entre os asmáticos obesos. Conclusões: Embora a leptina esteja envolvida na patogênese da asma em crianças asmáticas obesas ou não, seu efeito é maior naquelas obesas. Na presença de altos níveis de leptina, somente as crianças asmáticas obesas apresentaram polarização Th1 com maiores níveis de IFN- e asma mais grave.
Palavras-chave:
Leptina; Asma; Interferon gama; Interleucina-4.
IntroduçãoA asma consiste em uma doença inflamatória crônica das vias aéreas, estando associada à hiper-responsividade das vias aéreas que leva a recorrentes episódios de obstrução generalizada, e muitas vezes reversível, ao fluxo aéreo intrapulmonar.(1) Tornou-se claro que a obesidade está associada a um estado inflamatório sistêmico.(2) O aumento de tecido adiposo em sujeitos obesos produz um incremento das concentrações séricas de várias citocinas, quimiocinas e adipocinas pró-inflamatórias, o que também pode resultar em hiper-reatividade.(3)
A obesidade é um fator de risco para a asma.(4) A adiposidade pode aumentar a gravidade da asma e alterar a eficácia dos medicamentos padronizados para asma.(5) Embora os mecanismos subjacentes à relação entre a obesidade e a asma ainda não tenham sido totalmente estabelecidos, evidências experimentais sugerem que alterações nos hormônios derivados do tecido adiposo, tais como a adipocina leptina, entre outros fatores, estão implicadas.(6)
A leptina é um dos hormônios derivados do tecido adiposo reguladores de energia e um produto do gene da obesidade. A leptina circulante se correlaciona positivamente com o percentual de gordura corpórea e com a massa de gordura corpórea. Além dos efeitos primários da leptina na regulação de energia, que ela exerce inibindo a ingestão de alimentos e aumentando o gasto energético, verificou-se que ela tem um papel regulatório dentro do sistema imune.(7) A capacidade regulatória da leptina está associada não somente à imunidade adaptativa, mas também ao sistema inato.(6)
A leptina promove a produção de óxido nítrico e de citocinas pró-inflamatórias em macrófagos e monócitos.(8) Demonstrou-se que a leptina estimula a produção de TNF- e de IL-6 pelo tecido adiposo,(9) assim como a liberação de espécies reativas de oxigênio pelos neutrófilos.(10,11)
Como os receptores de leptina são expressos em linfócitos T, a leptina promove a sobrevivência e proliferação de linfócitos T e a produção de citocinas, embora os efeitos da leptina ocorram somente em linfócitos Th1, com aumento da produção de IFN-,(8) e não em linfócitos Th2, que também produzem citocinas.(12) A citocina IFN-, que é derivada predominantemente de células T e de células T "natural killer",(13) tem um papel importante nas respostas imunes inatas e adaptativas, particularmente em relação a infecções virais. Como a IFN- é secretada principalmente por células T e por células T "natural killer",(14) evidências emergentes sugerem que aumentos da população de células T no tecido adiposo e a modulação das células T pela leptina para uma resposta imune Th1, com aumento da produção de IFN-,(12,14) contribuem para a obesidade em sujeitos com um fenótipo específico da asma.
O objetivo do presente estudo foi avaliar os níveis de leptina sérica e seu efeito no equilíbrio Th1/Th2 por meio da medição da principal citocina Th1 (IFN-) e da principal citocina Th2 (IL-4) em crianças asmáticas obesas e não obesas, assim como investigar a associação desses níveis séricos com desfechos clínicos.
MétodosTrata-se de um estudo de caso-controle envolvendo 50 crianças atópicas com asma persistente moderada a grave e um grupo controle de 20 crianças não obesas não asmáticas. As crianças do primeiro grupo foram diagnosticadas com asma por um pneumologista pediátrico, de acordo com as diretrizes da Global Initiative for Asthma, pelos menos seis meses antes do início do estudo e tinham sido acompanhadas na Divisão de Asma e Alergia do Departamento de Pediatria do Complexo Hospitalar da Universidade de Zagazig, localizado na cidade do Cairo, Egito.
Os critérios de exclusão foram o uso de corticosteroides uma semana antes da coleta de sangue e o uso de medicamentos para asma, tais como broncodilatadores, 24 h antes da coleta de sangue. Além disso, foram excluídas as crianças com qualquer outra doença aguda ou crônica, incluindo infecção aguda do trato respiratório superior/inferior e doença cardíaca, cerebral ou endócrina, e aquelas com história de doença infecciosa nos últimos dois meses ou que tivessem usado qualquer medicamento na última semana.
As crianças asmáticas foram agrupadas como obesas e não obesas de acordo com o percentil do índice de massa corpórea (IMC). Definiu-se obesidade como um IMC, obtido por meio da equação peso (kg)/estatura2(m2), acima do percentil 95 para idade e sexo.(15) Todas as crianças diagnosticadas com asma preencheram os critérios diagnósticos para asma brônquica estabelecidos pela Global Initiative for Asthma.(16)
As características sociodemográficas das crianças, incluindo idade e sexo, foram registradas. Peso e estatura foram medidos com estadiômetro e balança eletrônica, respectivamente, sob a supervisão dos pesquisadores. Os escores de sintomas de asma foram avaliados e registrados para as crianças asmáticas. Foram determinados os níveis séricos de leptina, IL-4, IFN- e IgE. O VEF1 (% do previsto) também foi medido no dia da coleta das amostras.
O estudo foi aprovado pelo comitê de ética em pesquisa da universidade. Os pais ou responsáveis pelas crianças assinaram o termo de consentimento livre e esclarecido.
Os escores de sintomas nas crianças asmáticas foram avaliados de acordo com um escore de sintomas de asma constituído de seis domínios.(17) Os itens da escala incluíam dispneia, aperto no peito, sibilância durante o dia, sibilância à noite, e desempenho diário, pontuados de zero a três de acordo com a gravidade.
O uso de broncodilatadores foi suspenso antes do teste de função pulmonar (broncodilatadores de curta duração foram suspensos pelo menos 8 h antes do teste). Foi realizada espirometria dinâmica com um pneumotacógrafo (Masterlab Jaeger, Würzberg, Alemanha). O VEF1% foi determinado de acordo com os padrões da European Respiratory Society. O maior valor de VEF1 obtido em três manobras foi utilizado para a análise dos dados.
Para a medição dos níveis de leptina, IL-4 e IFN-, foram obtidas amostras de sangue periférico (2 mL) às 9h00, após uma noite de jejum. As amostras de sangue foram deixadas coagular por 60 min à temperatura ambiente. O soro foi separado por centrifugação a 1.200 g por 10 min e armazenado a −80°C. As amostras de soro foram descongeladas à temperatura ambiente antes das medições.
Os níveis séricos de leptina foram determinados por ELISA sanduíche duplo anticorpo com um anticorpo específico contra leptina humana (RD Systems, Minneapolis, MN, EUA). A sensibilidade do ensaio foi de 0,1 ng/mL, e o coeficiente de variação intraensaio foi de 3,6%. A dose mínima detectável de leptina é tipicamente < 7,8 pg/mL. O níveis séricos de lL-4 e IFN-, assim como os níveis de IgE total, foram medidos por ELISA (RD Systems). As amostras de soro foram armazenadas a −70°C até a realização do ensaio.
A análise estatística foi realizada utilizando-se o programa Statistical Package for the Social Sciences, versão 13.0 (SPSS Inc., Chicago, IL, EUA). Os dados foram expressos em média ± dp. A comparação dos parâmetros entre os grupos foi realizada utilizando-se one-way ANOVA. A análise de correlação de Pearson foi utilizada para avaliar a relação entre os parâmetros. O nível de significância adotado foi de p < 0,05.
ResultadosAs médias de idade das crianças asmáticas obesas (n = 25), crianças asmáticas não obesas (n = 25) e controles (n = 20) foram de 9,3 ± 2,5 anos, 10,4 ± 1,3 anos e 10,7 ± 2,9 anos, respectivamente (p = 0,06). O sexo masculino constituiu 60%, 55% e 45% dos respectivos grupos (p = 0,66; Tabela 1).
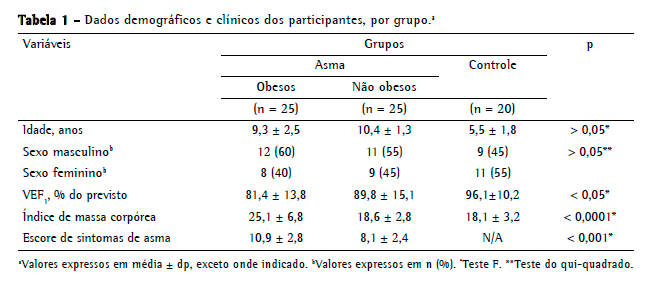
Como esperado, a média do IMC foi significativamente maior nos asmáticos obesos do que nos asmáticos não obesos e nos controles (p < 0,001; Tabela 1). A média do escore de sintomas de asma foi significativamente maior nos asmáticos obesos do que nos asmáticos não obesos (10,9 ± 2,8 vs. 8,1 ± 2,4; p < 0,001; Tabela 1).
A média do VEF1% foi significativamente menor nos asmáticos obesos do que nos asmáticos não obesos (81,4 ± 13,8% vs. 89,8 ± 15,1%; p < 0,005; Tabela 1).
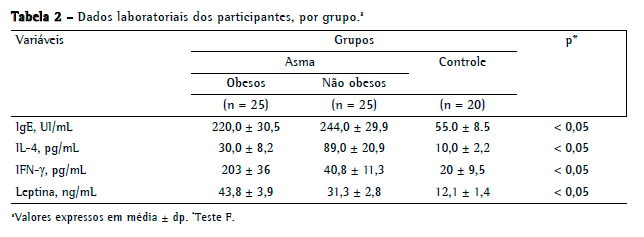
Os níveis médios de leptina diferiram significativamente entre os asmáticos obesos, os asmáticos não obesos e os controles (43,8 ± 3,9 pg/mL; 31,3 ± 2,8 pg/mL; e 12,1 ± 1,4 pg/mL, respectivamente; p < 0,05 para todos; Tabela 2), da mesma forma que os níveis médios de IFN- (203 ± 36 pg/mL; 40,8 ± 11,3 pg/mL; e 20,0 ± 9,5 pg/mL, respectivamente; p < 0,05 para todos) e os níveis médios de IL-4 (30,0 ± 8,2 pg/mL; 89,0 ± 20,9 pg/mL; e 10,0 ± 2,2 pg/mL; p < 0,05 para todos; Tabela 2).
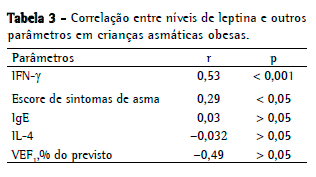
Nos asmáticos obesos, os níveis de leptina apresentaram correlações positivas significativas com os níveis de IFN- (r = 0,53) e com os escores de sintomas de asma (r = 0,29), assim como uma correlação negativa significativa com o VEF1% (r = −0,49). Entretanto, não houve correlação significativa entre os níveis de leptina e os de IL-4 (Tabela 3).
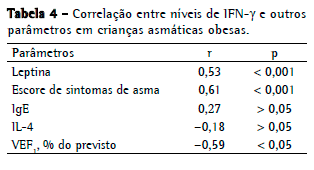
Nos asmáticos obesos, os níveis de IFN- apresentaram uma correlação positiva significativa com os escores de sintomas de asma (r = 0,61) e uma correlação negativa com o VEF1% (r = −0,59). Novamente, nenhuma correlação significativa foi encontrada entre os níveis de IFN- e os de IL-4 (Tabela 4). Nos asmáticos não obesos, os níveis de leptina se correlacionaram significativamente somente com os escores de sintomas de asma.
 Discussão
DiscussãoNo mundo moderno, há uma tendência de aumento da frequência de obesidade e asma.(18) A elucidação do mecanismo patogênico da associação entre essas duas entidades pode ajudar a resolver a falha terapêutica em crianças asmáticas obesas.(18) Recentemente, os hormônios e as citocinas liberadas pelo tecido adiposo têm sido foco de pesquisas sobre essa associação.(18)
A leptina, um hormônio do tecido adiposo, está associada à inflamação,(19) e demonstrou-se que seus níveis são maiores em crianças asmáticas do que em não asmáticas.(20) Entretanto, estudos sobre a relação entre a leptina e a asma em crianças produziram resultados inconsistentes.(3,20)
Observamos níveis elevados de leptina nas crianças asmáticas obesas e não obesas quando comparadas aos controles, e essa diferença foi mais acentuada nas crianças asmáticas obesas. Isso sugere que a leptina pode ter um papel de mediador inflamatório na asma, corroborando relatos anteriores sobre suas características inflamatórias.(21)
Em nosso estudo, o VEF1% foi menor e os escores de sintomas de asma foram maiores nas crianças asmáticas obesas do que nas crianças asmáticas não obesas. Isso sugere que os maiores níveis de leptina nas crianças asmáticas obesas podem ter contribuído para os maiores escores de sintomas e a função pulmonar reduzida em comparação às crianças asmáticas não obesas. Sin e Man demonstraram que altas concentrações de leptina estão associadas a comprometimento pulmonar em sujeitos obesos.(22)
No presente estudo, os níveis de leptina se correlacionaram positivamente com os escores de sintomas de asma nas crianças asmáticas obesas e não obesas e se correlacionaram negativamente com o VEF1% somente nas crianças asmáticas obesas. Isso indica que, além do efeito acentuado da leptina em crianças asmáticas obesas, a leptina tem também um papel na resposta inflamatória em crianças asmáticas não obesas.
Em um estudo clínico, Gurkan et al. demonstraram que, nas crianças asmáticas diagnosticadas recentemente (antes do tratamento com budesonida), os níveis séricos de leptina eram maiores do que nas crianças não asmáticas pareadas pelo IMC.(23) Guler et al. relataram que um aumento de um log nos níveis de leptina representou a duplicação da OR para asma após ajuste para IMC.(24)
Mai et al. estudaram a associação entre os níveis séricos de leptina e a asma em crianças com e sem sobrepeso na Suécia.(25) Constataram que os níveis séricos de leptina eram duas vezes maiores nas crianças com sobrepeso asmáticas do que nas crianças com sobrepeso não asmáticas. Nas crianças sem sobrepeso, entretanto, os autores constataram que os níveis séricos de leptina eram semelhantes entre as asmáticas e as não asmáticas. Portanto, com relação ao efeito interativo da leptina e da obesidade na asma, os dados favorecem a hipótese de que a leptina tem um papel importante na patogênese inflamatória da asma e de que esse papel tem um efeito mais acentuado em obesos com asma mais grave.
Além de sua função endócrina e metabólica, a leptina aumenta a resposta Th1, suprime as vias Th2 e pode exercer efeitos diretos na proliferação de linfócitos T CD4+ e na fagocitose de macrófagos. Em crianças obesas, a leptina promove a produção de IFN- em células "naïve" e em células T de memória, mas inibe a síntese de IL-4 em células T de memória.(26)
Constatamos que os níveis de IFN- estavam significativamente elevados e os níveis de IL-4 estavam reduzidos somente nas crianças asmáticas obesas, com uma correlação positiva significativa entre os níveis de IFN- e os níveis séricos de leptina. Assim, a inflamação das vias aéreas em crianças asmáticas obesas pode apresentar um padrão diferente com uma mudança para um perfil de citocinas Th1 dominado pela produção de IFN- e mediado pela leptina. Lord et al. e La Cava et al. demonstraram que as ações da leptina no sistema imune são complexas e que os níveis de IFN- são significativamente aumentados pela leptina.(26,27) Fernandez-Real et al. relataram que, em uma reação mista de linfócitos, a leptina favoreceu a diferenciação de células T para o fenótipo Th1.(28)
No presente estudo, os níveis de IFN- se correlacionaram positivamente com os escores de sintomas de asma e negativamente com o VEF1% somente nas crianças obesas. Nossos dados sugerem que, na presença de altos níveis de leptina, há um aumento da produção de IFN- pelas células polarizadas a Th1,(22,28) com agravamento da inflamação das vias aéreas e desfechos clínicos mais graves. Nosso dados são corroborados por Rastogi et al., que demonstraram que as crianças asmáticas obesas apresentaram menor relação VEF1/CVF do que as crianças asmáticas não obesas e as crianças não asmáticas, e que a relação VEF1/CVF se correlacionou negativamente com os níveis séricos de IFN-.(29) Além disso, observou-se uma maior proporção de células T CD4− secretoras de IFN- nas amostras de sangue de crianças obesas do que nas de crianças magras, e essa diferença se correlacionou com os níveis séricos de leptina. Nossos achados corroboram os de Yuksel et al., que concluíram que a leptina tem um papel na gravidade da asma em crianças.(30)
Nossos dados sustentam a hipótese de que pacientes asmáticos obesos apresentam polarização Th1, enquanto pacientes asmáticos magros apresentam polarização Th2, e de que o IFN- pode ser uma via no processo de inflamação induzida pela leptina. Isso sugere que, em asmáticos obesos, a polarização Th1 pode ser modulada mais pela obesidade do que pela própria asma, e isso é sustentado pela falta de correlação entre os níveis de leptina e de IFN-e entre os níveis de leptina e de IgE nas crianças asmáticas obesas.
Concluímos que, embora a leptina sérica esteja envolvida na patogênese da asma em crianças obesas e não obesas, seu efeito é mais acentuado em pacientes obesos. Na presença de altos níveis de leptina, somente as crianças asmáticas obesas apresentaram polarização Th1, com maiores níveis de IFN-.
Em crianças asmáticas obesas, a leptina e o IFN- agravam e aumentam a inflamação das vias aéreas, aumentando assim a gravidade da asma. Estratégias para o tratamento de crianças asmáticas obesas devem levar em conta esses achados.
Referências1. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143-78. http://dx.doi.org/10.1183/09031936.00138707 PMid:18166595
2. Mohamed GM, Hafez HM. Innate immunity in obese asthmatic allergic and nonallergic adults. Egypt J Immunol. 2009;16(2):1-8. PMid:22059348
3. Sood A. Obesity, adipokines, and lung disease. J Appl Physiol. 2010;108(3):744-53. http://dx.doi.org/10.1152/japplphysiol.00838.2009 PMid:19926824 PMCid:PMC2838636
4. Stream AR, Sutherland ER. Obesity and asthma disease phenotypes. Curr Opin Allergy Clin Immunol. 2012;12(1):76-81. http://dx.doi.org/10.1097/ACI.0b013e32834eca41 PMid:22157152
5. Barranco P, Delgado J, Gallego LT, Bobolea I, Pedrosa M, García de Lorenzo A, et al. Asthma, obesity and diet [Article in Spanish]. Nutr Hosp. 2012;27(1):138-45. PMid:22566313
6. Ziora D, Sitek P, Machura E, Ziora K. Bronchial asthma in obesity--a distinct phenotype of asthma? [Article in Polish]. Pneumonol Alergol Pol. 2012;80(5):454-62. PMid:22926907
7. Malli F, Papaioannou AI, Gourgoulianis KI, Daniil Z. The role of leptin in the respiratory system: an overview. Respir Res. 2010;11:152. http://dx.doi.org/10.1186/1465-9921-11-152 PMid:21040518 PMCid:PMC2988727
8. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911-9; quiz 920. http://dx.doi.org/10.1016/j.jaci.2005.02.023 PMid:15867843
9. Schwarzenberg SJ, Sinaiko AR. Obesity and inflammation in children. Paediatr Respir Rev. 2006;7(4):239-46. http://dx.doi.org/10.1016/j.prrv.2006.08.002 PMid:17098638
10. Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol. 2008;104(6):1727-35. http://dx.doi.org/10.1152/japplphysiol.00075.2008 PMid:18323466
11. Ali Assad N, Sood A. Leptin, adiponectin and pulmonary diseases. Biochimie. 2012;94(10):2180-9. http://dx.doi.org/10.1016/j.biochi.2012.03.006 PMid:22445899
12. Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176(12): 7745-52. PMid:16751422
13. Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836-45. http://dx.doi.org/10.4049/jimmunol.1000021 PMid:20581149
14. Wong N, Fam BC, Cempako GR, Steinberg GR, Walder K, Kay TW, et al. Deficiency in interferon-gamma results in reduced body weight and better glucose tolerance in mice. Endocrinology. 2011;152(10):3690-9. http://dx.doi.org/10.1210/en.2011-0288 PMid:21791564
15. Barlow SE, Dietz WH. Obesity evaluation and treatment: Expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998;102(3):E29. http://dx.doi.org/10.1542/peds.102.3.e29 PMid:9724677
16. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Bethesda: National Institutes of Health; 2002.
17. Yeh KH, Skowronski ME, Coreno AJ, Seitz RE, Villalba KD, Dickey-White H, et al. Impact of obesity on the severity and therapeutic responsiveness of acute episodes of asthma. J Asthma. 2011;48(6):546-52. http://dx.doi.org/10.3109/02770903.2011.581733 PMid:21604921
18. Kim KW, Shin YH, Lee KE, Kim ES, Sohn MH, Kim KE. Relationship between adipokines and manifestations of childhood asthma. Pediatr Allergy Immunol. 2008;19(6):535-40. http://dx.doi.org/10.1111/j.1399-3038.2007.00690.x PMid:18221467
19. Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol. 2009;20(1):81-8. http://dx.doi.org/10.1111/j.1399-3038.2008.00740.x PMid:18331416
20. Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115(1):103-9. http://dx.doi.org/10.1016/j.jaci.2004.10.007 PMid:15637554
21. Mito N, Kitada C, Hosoda T, Sato K. Effect of diet-induced obesity on ovalbumin-specific immune response in a murine asthma model. Metabolism. 2002;51(10):1241-6. http://dx.doi.org/10.1053/meta.2002.35196 PMid:12370841
22. Sin DD, Man SF. Impaired lung function and serum leptin in men and women with normal body weight: a population based study. Thorax. 2003;58(8):695-8. http://dx.doi.org/10.1136/thorax.58.8.695 PMCid:PMC1746780
23. Gurkan F, Atamer Y, Ece A, Kocyigit Y, Tuzun H, Mete N. Serum leptin levels in asthmatic children treated with an inhaled corticosteroid. Ann Allergy Asthma Immunol. 2004;93(3):277-80. http://dx.doi.org/10.1016/S1081-1206(10)61501-3
24. Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004;114(2):254-9. http://dx.doi.org/10.1016/j.jaci.2004.03.053 PMid:15316499
25. Mai XM, Böttcher MF, Leijon I. Leptin and asthma in overweight children at 12 years of age. Pediatr Allergy Immunol. 2004;15(6):523-30. http://dx.doi.org/10.1111/j.1399-3038.2004.00195.x PMid:15610366
26. Lord GM, Matarese G, Howard JK, Bloom SR, Lechler RI. Leptin inhibits the anti-CD3-driven proliferation of peripheral blood T cells but enhances the production of proinflammatory cytokines. J Leukoc Biol. 2002;72(2):330-8. PMid:12149424
27. La Cava A, Alviggi C, Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J Mol Med (Berl). 2004;82(1):4-11. http://dx.doi.org/10.1007/s00109-003-0492-1 PMid:14556053
28. Fernandez-Real JM, Broch M, Vendrell J, Ricart W. Smoking, fat mass and activation of the tumor necrosis factor-alpha pathway. Int J Obes Relat Metab Disord. 2003;27(12):1552-6. http://dx.doi.org/10.1038/sj.ijo.0802472 PMid:12975637
29. Rastogi D, Canfield SM, Andrade A, Isasi CR, Hall CB, Rubinstein A, et al. Obesity-associated asthma in children: a distinct entity. Chest. 2012;141(4):895-905. http://dx.doi.org/10.1378/chest.11-0930 PMid:21980061
30. Yuksel H, Sogut A, Yilmaz O, Onur E, Dinc G. Role of adipokines and hormones of obesity in childhood asthma. Allergy Asthma Immunol Res. 2012;4(2):98-103. http://dx.doi.org/10.4168/aair.2012.4.2.98 PMid:22379605 PMCid:PMC3283800
* Trabalho realizado na Universidade de Zagazig, Cairo, Egito.
Endereço para correspondência: Doaa Youssef. Zagazig University, 310 Zahraa Nasr City, 11231, Cairo, Egypt.
Tel. 201222839220. E-mail: dody5176@yahoo.com
Apoio financeiro: Nenhum.
Recebido para publicação em 6/5/2013. Aprovado, após revisão, em 5/9/2013.
Sobre os autoresDoaa Mohammed Youssef
Professor Assistente de Pediatria. Universidade de Zagazig, Cairo, Egito.
Rabab Mohamed Elbehidy
Professora Assistente de Pediatria. Universidade de Zagazig, Cairo, Egito.
Dina Mahamoud Shokry
Professora Assistente de Pediatria. Universidade de Zagazig, Cairo, Egito.
Eman Mohamed Elbehidy
Professora Assistente de Microbiologia e Imunologia. Universidade de Zagazig, Cairo, Egito.







