ABSTRACT
Objective: Patients with high cervical spinal cord injury are usually dependent on mechanical ventilation support, which, albeit life saving, is associated with complications and decreased life expectancy because of respiratory infections. Diaphragm pacing stimulation (DPS), sometimes referred to as electric ventilation, induces inhalation by stimulating the inspiratory muscles. Our objective was to highlight the indications for and some aspects of the surgical technique employed in the laparoscopic insertion of the DPS electrodes, as well as to describe five cases of tetraplegic patients submitted to the technique. Methods: Patient selection involved transcutaneous phrenic nerve studies in order to determine whether the phrenic nerves were preserved. The surgical approach was traditional laparoscopy, with four ports. The initial step was electrical mapping in order to locate the "motor points" (the points at which stimulation would cause maximal contraction of the diaphragm). If the diaphragm mapping was successful, four electrodes were implanted into the abdominal surface of the diaphragm, two on each side, to stimulate the branches of the phrenic nerve. Results: Of the five patients, three could breathe using DPS alone for more than 24 h, one could do so for more than 6 h, and one could not do so at all. Conclusions: Although a longer follow-up period is needed in order to reach definitive conclusions, the initial results have been promising. At this writing, most of our patients have been able to remain ventilator-free for long periods of time.
Keywords:
Spinal cord injuries; Quadriplegia; Respiration, artificial; Pacemaker, artificial; Diaphragm.
RESUMO
Objetivo: Pacientes com lesão medular cervical alta em geral são dependentes de ventilação mecânica, que, embora salve vidas, está associada a complicações e redução da expectativa de vida devido a infecções respiratórias. A estimulação do diafragma por marca-passo, às vezes chamada de ventilação elétrica, induz a inspiração por estimulação dos músculos inspiratórios. Nosso objetivo foi destacar as indicações e alguns aspectos da técnica cirúrgica empregada no implante laparoscópico dos eletrodos, assim como descrever cinco casos de pacientes tetraplégicos submetidos à técnica. Métodos: A seleção dos pacientes envolveu estudos de condução do nervo frênico por via transcutânea para determinar se os nervos estavam preservados. A abordagem cirúrgica foi laparoscopia clássica, com quatro trocartes. A técnica foi iniciada com o mapeamento elétrico para encontrar os "pontos motores" (pontos de contração máxima do diafragma). Se o mapeamento era bem-sucedido, dois eletrodos eram implantados na face abdominal de cada lado do diafragma para estimular ramos do nervo frênico. Resultados: Dos cinco pacientes, três e um, respectivamente, eram capazes de respirar somente com o uso do marca-passo por períodos superiores a 24 e 6 h, enquanto um não era capaz. Conclusões: Embora seja necessário um acompanhamento mais longo para chegar a conclusões definitivas, os resultados iniciais são promissores, pois, no momento, a maioria dos nossos pacientes pode permanecer sem ventilação mecânica por longos períodos de tempo.
Palavras-chave:
Traumatismos da medula espinal; Quadriplegia; Respiração artificial; Marca-passo artificial; Diafragma.
IntroductionPatients with high cervical spinal cord injury (SCI) are usually dependent on mechanical ventilation (MV) support, which, despite being life saving, is associated with a myriad of significant complications, such as increased secretions, difficulty with speech, constant noise, increased anxiety, loss of smell, and inability to find living facilities or nursing care. In addition, patients using ventilators have a significant reduction in their life expectancy because of respiratory infections, mainly caused by poor ventilation of the posterior lobe. From an economic standpoint, the average annual health care and living expenses due to ventilator dependence in tetraplegic patients in the United States are more than US$ 170,000.(1)
Phrenic nerve pacing has been used with mixed success for over 40 years in ventilator-dependent patients.(2) However, a recent long-term follow-up study has demonstrated a reduction in respiratory infections, improved cost-benefit ratio, and improved quality of life in those submitted to diaphragm pacing stimulation (DPS).(3)
In the past, the traditional method of phrenic nerve pacing required bilateral cervical exploration or thoracotomy followed by phrenic nerve dissection and mobilization.(4,5) The implantation of the electrodes at these sites can be difficult, and, if the phrenic nerve is injured, the diaphragm will be nonfunctional. However, the more recent approach to DPS (i.e., via laparoscopy) avoids bilateral thoracotomy, allows the evaluation of the diaphragm in real time, and permits the intramuscular implantation of the electrodes into the motor points of the branches of the right and left hemidiaphragms.(6)
Electric ventilation, most commonly known as DPS, is the production of inhalation by applying rhythmic stimulation to the motor nerves and inspiratory muscles. It is a technique designed to replace, delay, or reduce the need for MV by natural negative pressure ventilation using the diaphragm of the patient. However, phrenic nerve function needs to be preserved for DPS to be successful.(6,7)
In addition to being used in patients with SCI or congenital central hypoventilation syndrome, the laparoscopic approach to DPS has been used in patients with motor neuron disease or amyotrophic lateral sclerosis (ALS),(8) as well as in difficult-to-wean patients in the ICU.(9)
The indications for DPS vary depending on the case. In patients with SCI, DPS is used when there is respiratory insufficiency due to the injury, whereas, in patients with ALS, DPS is used when the respiratory symptoms begin.(10)
We have recently developed a funded pilot project for diaphragm pacing via intramuscular diaphragm electrode implantation in patients who have become ventilator-dependent after SCI.(11) To the best of our knowledge, these are the first reported cases of intramuscular DPS in Latin America. The objective of the present study was to highlight the indications for and some aspects of the surgical technique employed in the laparoscopic insertion of the DPS electrodes. Here, we describe the most current version of the laparoscopic insertion technique and the results of the modifications that were made over the course of more than 500 implantation procedures performed worldwide. We hope that by improving the understanding of the procedure the present study can be used not only as a guide for surgeons involved in implantation procedures but also as a tool for nonsurgeons taking care of such patients.
MethodsBetween October and November of 2011, five patients who had suffered high cervical SCI (C4 or higher) and who were on long-term ventilation were evaluated to determine whether they could undergo diaphragm pacing with the DPS system (NeuRx®; Synapse Biomedical, Oberlin, OH, USA), in a trial conducted in the Thoracic Surgery and Neurosurgery Departments of the University of São Paulo School of Medicine Hospital das Clínicas Heart Institute, located in the city of São Paulo, Brazil. All of the patients gave written informed consent, and the study was approved by the research ethics committee of the institution (Process no. 0551/10).
Specific investigations were carried out for patient selection, including chest X-rays and transcutaneous phrenic nerve studies in order to determine whether the phrenic nerves were preserved. The characteristics of the patients, including gender, age, level of SCI, duration of MV, electromyographic response of the right and left phrenic nerves (as determined by electroneurography), and degree of ventilator dependence, are summarized in Table 1.
The anesthetic technique employed has been described in one study.(12) All of the patients selected had permanent tracheostomies, arrived at the operating room connected to their usual ventilator, and were transferred to the anesthesia ventilator (the settings of which were the same as those of the mechanical ventilator). This process was reversed at the end of the surgical procedure. The patients were monitored by electrocardiography, oximetry, and noninvasive arterial pressure measurement.
The surgical approach was traditional laparoscopy, with pneumoperitoneum and four ports. The patients were placed in the supine position, with arms outstretched, and elevated 30° during most of the procedure. Exposure of the diaphragm started with the placement of one 10-mm supraumbilical port and two 5-mm ports at the right and left subcostal incisions. After the falciform ligament was excised, one 12-mm subxiphoid port was placed.
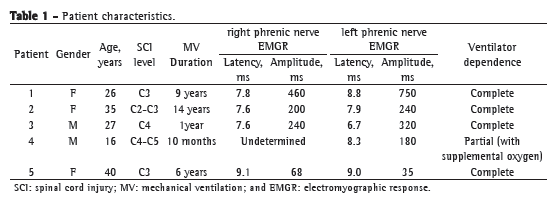
The initial step was electrical mapping in order to locate the points at which stimulation would cause maximal contraction of the diaphragm. Diaphragm mapping was the key step in the procedure, given that it shows where the DPS electrodes should be implanted. A standard Maryland dissector, coupled to the Clinical Station (Synapse Biomedical, Oberlin, OH, USA) by an alligator clip, was used in order to perform the mapping. The mapping technique involves working in parallel lines to the central tendinous portion of the diaphragm. After one line was completed, the surgeon moved upward away from the central tendon in order to identify areas of maximal contraction, which are usually close to the motor points (Figure 1).
Qualitative assessment was based on the degree of diaphragm contraction, as seen intraoperatively. Quantitative assessment of intra-abdominal pressure changes was performed with a tube that was attached to one of the laparoscopic ports, which in turn was attached to a pressure transducer.
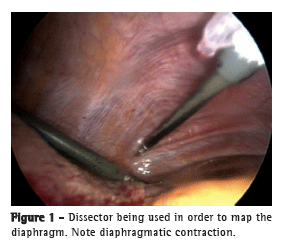
If the diaphragm mapping was successful, four electrodes were implanted into the abdominal surface of the diaphragm, two on each side, in order to stimulate the branches of the phrenic nerve. The implantation device was passed through the subxiphoid midline port. This allowed easy access to both hemidiaphragms. The laparoscopic implant instrument is retractable, with a hollow bore needle that is capable of angulations and full retraction. It allows safe, dependable, and accurate positioning of the electrode in the commonly thin wall of the atrophied diaphragm muscle (Figure 2).
After the electrodes had been implanted, Maryland dissectors were used in order to hold the wires in the abdomen as the implantation device was backed out through the midline port. Those pacing wires were passed out percutaneously and attached to the external pacing device in a manner somewhat similar to epicardial pacing after cardiac surgery.(6,7,13,14)
The Clinical Station that was used for electrode stimulation during the surgical procedure was also used in order to program the pacing unit to maximize patient ventilation. This unit allowed pacing to be turned on and off and provided the stimulus at the required amplitude and frequency (Figure 3).
The DPS electrodes were then attached to the clinical station, and the diaphragm was tested to confirm that the electrodes had been correctly positioned. The tail of the first electrode was brought back into the abdomen prior to the implantation of the second electrode on the same side. The tail of the second electrode was subsequently passed entirely into the abdomen, and the procedure was transitioned to the opposite side. The four electrodes were withdrawn through the subxiphoid port, care being taken to ensure that the right and left side wires were not inadvertently crossed.
The wire routing process involves tunneling the implanted electrodes from the subxiphoid exit site to a laterally located site on the skin. Four separate tunnels were created with the tunneling devices, one for each implanted electrode. An additional ground electrode was implanted at a remote location with a separate tunneling device. The tunneling devices were flushed with saline to ensure that they were free of tissue, and the electrodes were passed through each of the tunneling devices, in a pattern according to their location in the diaphragm, with an electrode coupling device (Figure 4).
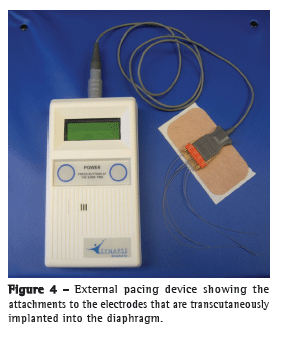
With the electrodes tunneled in their appropriate positions, the excess slack was pulled back into the abdominal cavity with the Maryland dissector. This was carefully done in order to prevent inadvertent placement of the electrodes exceedingly back into the abdominal cavity, pulling the exposed portion of each electrode back into the subcutaneous tunnel, the electrodes therefore becoming irretrievable.
The laparoscopic ports were withdrawn under direct visualization, fascia and skin incisions were closed, and the wounds were dressed. Gold pin connectors were attached to the ends of the electrodes, which were subsequently inserted into the connector block. The connector block was secured to the skin by a customized connector holder. The patient was again connected to the computer stimulator to ensure that all electrical connections were working.
ResultsOf the five patients selected, two presented with capnothorax related to intra-abdominal carbon dioxide insufflation (carbon dioxide pneumothorax). One of the patients developed bilateral carbon dioxide pneumothorax during anesthesia; this was rapidly diagnosed by evaluation of the pulmonary pressures. The patient required higher pressures for ventilation, which were difficult to achieve because the patient had an uncuffed endotracheal tube. The problem was quickly solved by puncturing the right side in order to drain the carbon dioxide pneumothorax and deflate the pneumoperitoneum. In the other patient, the carbon dioxide pneumothorax was detected by a postoperative chest X-ray. We decided to perform pleural drainage using a pigtail catheter. The chest tube was removed the following day.
During the surgical procedure, in one patient with elevated hemidiaphragm, a Valsalva maneuver, performed in order to produce downward force to help insert the permanent electrode, produced hypotension. The complication resolved with the interruption of the maneuver.
Six months after the procedures, three of the five patients were able to use DPS continuously. Another patient, who had been ventilator-dependent for 14 years, was able to use DPS 6 h a day. However, stimulation had to be interrupted after the onset of neuropathic pain that remained uncontrolled at this writing. The fifth patient, with the weakest electromyographic response, was unable to sustain ventilation with the DPS system.
DiscussionBecause the five patients had been tracheostomized, access to the airways was not a problem for the anesthesiologists. However, one of the patients had an uncuffed endotracheal tube, which jeopardized ventilation when capnothorax occurred (because of the air leak around the tube). Although ventilation can be maintained with an uncuffed tube, we recommend that it be replaced by a cuffed tube prior to the surgical procedure because of the risk of capnothorax.
Two patients presented with capnothorax, a finding that has been reported in the literature. In a recent report of SCI patients implanted with the DPS system,(6) the most common complication was capnothorax, which was seen in 42%. However, the capnothorax had clinical consequences in only 4%. Although electrode infection and migration have been reported,(6) they are uncommon; none of our patients had these complications.
Diaphragm mapping is the key step in the implantation procedure, and it is the step that has changed the most over time. Previously, a suction electrode catheter was used in order to identify points of contraction; currently, a standard Maryland dissector is used in order to do the mapping. The latter method has proven to be easy, reproducible, and faster than the former.
During diaphragm mapping, it is advisable to stimulate additional abdominal wall musculature if the diaphragm shows weak stimulation, in order to confirm that the electrode and the computer-based stimulator are working properly.
During diaphragm pacing, it is important to confirm whether there is no electrical interference that might affect the cardiac rhythm. Right and left side DPS electrodes should be tested. Subsequently, the four electrodes should be tested with the stimulator at maximum output settings. In addition to these tests, we used electrocardiography in order to confirm that there was no electrical interference. If interference is observed, DPS can be isolated to a single electrode, and appropriate modifications can be made by moving the electrode or programming stimulation to lower levels.
Another point of concern is the use of DPS and cardiac pacing. The use of DPS and cardiac pacemakers has been shown to be safe. The interaction between the devices can be clearly identified and avoided by changing the programming or the placement of the intramuscular electrodes. Because such patients present with increased cardiac susceptibility, which caused them to have a cardiac pacemaker in the first place, they should be treated with extra caution. Any increases in the DPS settings should be carried out in a monitored setting to ensure that there is no cardiac rhythm capture, as is the case during the initial electrode implantation in all patients.(15)
One group of authors, postulating that sleep-related disturbances are early clues to diaphragmatic dysfunction and therefore can provide a sensitive marker, assessed the effects of DPS on FVC.(10) After four months of diaphragm conditioning, patients with ALS exhibited significant sleep improvements. It is possible that the number of SCI or ALS patients submitted to DPS will increase in the future.(10)
Although a longer follow-up period is necessary in order to reach definitive conclusions, the initial results have been promising, since most of our patients could remain ventilator-free for long periods of time at this writing. Of the five patients, three could breathe using DPS alone for more than 24 h.
In conclusion, the implementation of preoperative testing, together with the surgical technique employed and the postoperative recovery protocol, does not require any extraordinary preparations or follow-up for the implantation of the electrodes and the DPS system. This is a procedure that can be readily repeated in a cost-effective manner. This is an important finding especially for patients with SCI, since the DPS procedure is usually more challenging in those patients than in ALS patients.
References1. National Spinal Cord Injury Statistical Center [homepage on the Internet]. Birmingham: The University of Alabama at Birmingham. [cited 2012 Jun 4]. Spinal Cord Injury Facts and Figures at a Glance February 2011. [Adobe Acrobat document, 2p.]. Available from: https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202012%20Feb%20Final.pdf
2. Miko I, Gould R, Wolf S, Afifi S. Acute spinal cord injury. Int Anesthesiol Clin. 2009;47(1):37-54. PMid:19131751. http://dx.doi.org/10.1097/AIA.0b013e3181950068
3. Hirschfeld S, Exner G, Luukkaala T, Baer GA. Mechanical ventilation or phrenic nerve stimulation for treatment of spinal cord injury-induced respiratory insufficiency. Spinal Cord. 2008;46(11):738-42. PMid:18475279. http://dx.doi.org/10.1038/sc.2008.43
4. Glenn WW, Hogan JF, Loke JS, Ciesielski TE, Phelps ML, Rowedder R. Ventilatory support by pacing of the conditioned diaphragm in quadriplegia. N Engl J Med. 1984;310(18):1150-5. PMid:6608692. http://dx.doi.org/10.1056/NEJM198405033101804
5. DiMarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT. Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med. 2002;166(12 Pt 1):1604-6. PMid:12471076. http://dx.doi.org/10.1164/rccm.200203-175CR
6. Onders RP, Elmo M, Khansarinia S, Bowman B, Yee J, Road J, et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc. 2009;23(7):1433 40. PMid:19067067. http://dx.doi.org/10.1007/s00464-008-0223-3
7. Onders RP, DiMarco AF, Ignagni AR, Mortimer JT. The learning curve for investigational surgery: lessons learned from laparoscopic diaphragm pacing for chronic ventilator dependence. Surg Endosc. 2005;19(5):633-7. PMid:15776209. http://dx.doi.org/10.1007/s00464-004-8934-6
8. Onders RP, Carlin AM, Elmo M, Sivashankaran S, Katirji B, Schilz R. Amyotrophic lateral sclerosis: the Midwestern surgical experience with the diaphragm pacing stimulation system shows that general anesthesia can be safely performed. Am J Surg. 2009;197(3):386 90. PMid:19245920. http://dx.doi.org/10.1016/j.amjsurg.2008.11.008
9. Onders R, McGee MF, Marks J, Chak A, Schilz R, Rosen MJ, et al. Diaphragm pacing with natural orifice transluminal endoscopic surgery: potential for difficult-to-wean intensive care unit patients. Surg Endosc. 2007;21(3):475-9. PMid:17177078. http://dx.doi.org/10.1007/s00464-006-9125-4
10. Gonzalez-Bermejo J, Morélot-Panzini C, Salachas F, Redolfi S, Straus C, Becquemin MH, et al. Diaphragm pacing improves sleep in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13(1):44-54. PMid:22023158. http://dx.doi.org/10.3109/17482968.2011.597862
11. ClinicalTrials.gov [homepage on the Internet]. Bethesda: U.S. National Institutes of Health. [cited 2012 Jun 4]. Diaphragmatic Pacemaker in Tetraplegic Patients With Spinal Cord Injuries. Available from: http://clinicaltrials.gov/ct2/show/NCT01385384?term=tedderank=1
12. Tedde ML, Vasconcelos-Filho P, Hajjar LA, Almeida JP, Flora GF, Okumura EM, et al. Diaphragmatic pacing stimulation in spinal cord injury: anesthetic and perioperative management. Clinics (Sao Paulo). In press 2012.
13. Onders RP, Aiyar H, Mortimer JT. Characterization of the human diaphragm muscle with respect to the phrenic nerve motor points for diaphragmatic pacing. Am Surg. 2004;70(3):241-7; discussion 247. PMid:15055848.
14. Reade MC. Temporary epicardial pacing after cardiac surgery: a practical review: part 1: general considerations in the management of epicardial pacing. Anaesthesia. 2007;62(3):264-71. Erratum in: Anaesthesia. 2007;62(6):644. PMid:17300304. http://dx.doi.org/10.1111/j.1365-2044.2007.04950.x
15. Onders RP, Khansarinia S, Weiser T, Chin C, Hungness E, Soper N, et al. Multicenter analysis of diaphragm pacing in tetraplegics with cardiac pacemakers: positive implications for ventilator weaning in intensive care units. Surgery. 2010;148(4):893-7; discussion 897-8. PMid:20797750. http://dx.doi.org/10.1016/j.surg.2010.07.008
Study carried out in the Department of Thoracic Surgery, Instituto do Coração - InCor, Heart Institute - University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil.
Correspondence to: Miguel L. Tedde. Rua Itambé, 367, apto. 151A, Higienópolis, CEP 01239-001, São Paulo, SP, Brasil.
Tel. 55 11 99653-5030. Fax: 55 11 2661-5197. E-mail: tedde@usp.br
Financial support: This study received financial support from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant no. 2010/50785-6) and from Synapse Biomedical International.
Submitted: 25 June 2012. Accepted, after review: 12 July 2012.
A versão completa em português deste artigo está disponível em www.jornaldepneumologia.com.br
About the authorsMiguel Lia Tedde
Attending Physician. Department of Thoracic Surgery, Instituto do Coração - InCor, Heart Institute - University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil.
Raymond P Onders
Director. Department of Minimally Invasive Surgery, University Hospitals Case Medical Center, Case Western Reserve University School of Medicine, Cleveland (OH) USA.
Manoel Jacobsen Teixeira
Full Professor. Department of Neurosurgery, Laboratório de Investigação Médica 26 (LIM-26, Laboratory for Medical Research 26), University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil.
Silvia Gelas Lage
Tenured Professor. Instituto do Coração - InCor, Heart Institute - University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil.
Gerson Ballester
Physician. Department of Neurosurgery, Laboratório de Investigação Médica 26 (LIM-26, Laboratory for Medical Research 26), University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil.
Mario Wilson Iersolino Brotto
Physician. Department of Neurology, University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil.
Erica Mie Okumura
Physiotherapist. Department of Thoracic Surgery, Instituto do Coração - InCor, Heart Institute - University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil.
Fabio Biscegli Jatene
Full Professor. Department of Thoracic Surgery, Instituto do Coração - InCor, Heart Institute - University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil.






